Exam 7: The Electronic Structure of Atoms
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
An AM radio station broadcasts at a frequency of 1270 kHz. Calculate the wavelength of the broadcast signal in meters. (c = 2.9979 * 108 m/s)
(Short Answer)
4.8/5  (37)
(37)
Calculate the wavelength of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level. Recall that for hydrogen En = -2.18 * 10-18 J(1/n2)
(Multiple Choice)
4.9/5  (44)
(44)
The colors of the visible spectrum are blue, green, orange, red, violet, and yellow. Of these colors, ______ has the least energy.
(Short Answer)
4.9/5  (29)
(29)
Which ground-state atom has an electron configuration described by the following orbital diagram? 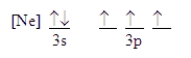
(Multiple Choice)
4.8/5  (43)
(43)
What is the wavelength, in meters, of an alpha particle with a kinetic energy of 8.0 * 10-13 J? (mass of an alpha particle = 4.00150 amu; 1 amu = 1.67 * 10-27 kg)
(Essay)
4.9/5  (38)
(38)
If a hydrogen atom and a helium atom have the same kinetic energy,
(Multiple Choice)
4.9/5  (33)
(33)
Breaking the oxygen-oxygen bond in hydrogen peroxide requires 210 kJ/mol. What is the longest wavelength of light that can cause this bond to be broken?
(Multiple Choice)
4.8/5  (31)
(31)
Which element has the following ground-state electron configuration? [Kr]5s14d5
(Multiple Choice)
4.8/5  (42)
(42)
A possible set of quantum numbers for the last electron added to complete an atom of gallium (Ga)in its ground state is 
(Multiple Choice)
4.8/5  (41)
(41)
Which choice lists two elements with ground-state electron configurations that are well known exceptions to the Aufbau principle?
(Multiple Choice)
4.8/5  (35)
(35)
The second line of the Balmer series occurs at a wavelength of 486.1 nm. What is the energy difference between the initial and final levels of the hydrogen atom in this emission process?
(Multiple Choice)
4.8/5  (36)
(36)
What is the total number of electrons possible in the 2p orbitals?
(Short Answer)
5.0/5  (42)
(42)
What is the maximum number of electrons in an atom that can have the following set of quantum numbers? n = 4 l = 3 ml = -2 ms = +1/2
(Multiple Choice)
4.8/5  (39)
(39)
An electron in a 3p orbital could have a value of 2 for its angular momentum quantum number (l).
(True/False)
4.9/5  (30)
(30)
Showing 61 - 80 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)