Exam 16: Electrical Energy and Capacitance: Part A
Exam 1: Introduction60 Questions
Exam 1: Introduction: Part A47 Questions
Exam 2: Motion in One Dimension64 Questions
Exam 2: Motion in One Dimension: Part A42 Questions
Exam 3: Vectors and Two-Dimensional Motion74 Questions
Exam 3: Vectors and Two-Dimensional Motion: Part A64 Questions
Exam 4: The Laws of Motion93 Questions
Exam 4: The Laws of Motion: Part A69 Questions
Exam 5: Energy84 Questions
Exam 5: Energy: Part A32 Questions
Exam 6: Momentum and Collisions83 Questions
Exam 6: Momentum and Collisions: Part A61 Questions
Exam 7: Rotational Motion and the Law of Gravity84 Questions
Exam 7: Rotational Motion and the Law of Gravity: Part A48 Questions
Exam 8: Rotational Equilibrium and Rotational Dynamics60 Questions
Exam 8: Rotational Equilibrium and Rotational Dynamics: Part A61 Questions
Exam 9: Solids and Fluids78 Questions
Exam 9: Solids and Fluids: Part A46 Questions
Exam 10: Thermal Physics82 Questions
Exam 10: Thermal Physics: Part A56 Questions
Exam 11: Energy in Thermal Processes Heat and Internal Energy82 Questions
Exam 11: Energy in Thermal Processes Heat and Internal Energy: Part A54 Questions
Exam 12: The Laws of Thermodynamics Work in Thermodynamic Processes70 Questions
Exam 12: The Laws of Thermodynamics Work in Thermodynamic Processes: Part A40 Questions
Exam 13: Vibrations and Waves83 Questions
Exam 13: Vibrations and Waves: Part A48 Questions
Exam 14: Sound81 Questions
Exam 14: Sound: Part A67 Questions
Exam 15: Electric Forces and Electric Fields81 Questions
Exam 15: Electric Forces and Electric Fields: Part A42 Questions
Exam 16: Electrical Energy and Capacitance81 Questions
Exam 16: Electrical Energy and Capacitance: Part A33 Questions
Exam 17: Current and Resistance Electric Current83 Questions
Exam 17: Current and Resistance Electric Current: Part A37 Questions
Exam 18: Direct-Current Circuits Sources of EMF77 Questions
Exam 18: Direct-Current Circuits Sources of EMF: Part A54 Questions
Exam 19: Magnetism Magnets82 Questions
Exam 19: Magnetism Magnets: Part A67 Questions
Exam 20: Induced Voltages and Inductance83 Questions
Exam 20: Induced Voltages and Inductance: Part A46 Questions
Exam 21: Alternating-Current Circuits and Electromagnetic Waves98 Questions
Exam 21: Alternating-Current Circuits and Electromagnetic Waves: Part A32 Questions
Exam 22: Reflection and Refraction of Light81 Questions
Exam 22: Reflection and Refraction of Light: Part A49 Questions
Exam 23: Mirrors and Lenses82 Questions
Exam 23: Mirrors and Lenses: Part A31 Questions
Exam 24: Wave Optics88 Questions
Exam 24: Wave Optics: Part A71 Questions
Exam 25: Optical Instruments79 Questions
Exam 25: Optical Instruments: Part A59 Questions
Exam 26: Relativity62 Questions
Exam 26: Relativity: Part A29 Questions
Exam 27: Quantum Physics Blackbody Radiation and Plancks Hypothesis79 Questions
Exam 27: Quantum Physics Blackbody Radiation and Plancks Hypothesis: Part A52 Questions
Exam 28: Atomic Physics71 Questions
Exam 28: Atomic Physics: Part A38 Questions
Exam 29: Nuclear Physics75 Questions
Exam 29: Nuclear Physics: Part A43 Questions
Exam 30: Nuclear Energy and Elementary Particles88 Questions
Exam 30: Nuclear Energy and Elementary Particles: Part A37 Questions
Exam 31: Particle Collisions, Mediating Photons, and Quark Structures: Exploring the Fundamentals of Physics16 Questions
Select questions type
A closed 2.0-L container holds 3.0 mol of an ideal gas.If 150 J of heat is added,what is the change in internal energy of the system?
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
C
Three Carnot engines operate between temperature reservoirs as follows: Engine A: Th = 1300 K,Tc = 1000 K;Engine B: Th = 1000 K,Tc = 700 K;Engine C: Th = 500 K,Tc = 350 K.Which two engines have the same thermal efficiency?
Free
(Multiple Choice)
5.0/5  (43)
(43)
Correct Answer:
B
When gasoline is burned,it gives off 46,000 J/g of heat energy.If an automobile uses 13.0 kg of gasoline per hour with an efficiency of 18%,what is the average horsepower output of the engine? (1 hp = 746 W)
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
D
During each cycle of operation a refrigerator absorbs 45 cal from the freezer compartment and expels 85 cal to the room.If one cycle occurs every 8.0 s,how many minutes will it take to freeze 500 g of water,initially at 0°C? (Lv = 80 cal/g)
(Multiple Choice)
4.7/5  (36)
(36)
A heat engine exhausts 4000 J of heat while performing 2000 J of useful work.What is the efficiency of the engine?
(Multiple Choice)
4.9/5  (35)
(35)
An avalanche of ice and snow of mass 2500 kg slides a vertical distance of 160 m down a mountainside.If the temperature of the ice,snow,mountain,and surrounding air are all at 0°C,what is the change in entropy of the universe?
(Multiple Choice)
4.9/5  (39)
(39)
The laws of thermodynamics can be related to the following: (i)the measurement of temperature, (ii)the lowest temperature possible, (iii)conservation of energy,and (iv)only part of heat energy available may be turned into work.Which of these involve the zeroth and third law?
(Multiple Choice)
4.7/5  (36)
(36)
An electrical generating plant operates at a boiler temperature of 200°C and exhausts the unused heat into a nearby river at 20°C.What is the maximum theoretical efficiency of the plant? (0°C = 273 K)
(Multiple Choice)
4.8/5  (32)
(32)
A heat engine operating between a pair of hot and cold reservoirs with respective temperatures of 500 K and 300 K will have what maximum efficiency?
(Multiple Choice)
4.8/5  (32)
(32)
The surface of the Sun is at approximately 5700 K,and the temperature of the Earth's surface is about 290 K.What total entropy change occurs when 2000 J of heat energy is transferred from the Sun to the Earth?
(Multiple Choice)
4.8/5  (31)
(31)
A 2.0-mol ideal gas system is maintained at a constant volume of 4.0 L.If 200 J of heat is added,what is the work done on the system?
(Multiple Choice)
4.7/5  (29)
(29)
In an isobaric process 3.6 × 104 J of work is done on a quantity of gas while its volume changes from 2.3 m3 to 1.1 m3.What is the pressure during this process?
(Multiple Choice)
4.8/5  (34)
(34)
You have a PV diagram for a device going through a cyclic process forming a closed loop on the diagram.How can you tell from the diagram whether the device is acting as a heat engine and not as a refrigerator?
(Multiple Choice)
4.8/5  (45)
(45)
In an isothermal process where the pressure decreases from 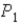 To
To 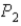 ,does the internal energy of the system increase,decrease,or stay the same;and does the entropy of the system increase or decrease?
,does the internal energy of the system increase,decrease,or stay the same;and does the entropy of the system increase or decrease?
(Multiple Choice)
4.9/5  (29)
(29)
The laws of thermodynamics can be related to the following: (i)the measurement of temperature, (ii)the lowest temperature possible, (iii)conservation of energy,and (iv)only part of heat energy available may be turned into work.Which of these involve the first and second law?
(Multiple Choice)
4.7/5  (30)
(30)
A quantity of monatomic ideal gas expands adiabatically from a volume of 2.0 liters to 4.0 liters.If the initial pressure is P0,what is the final pressure?
(Multiple Choice)
4.8/5  (29)
(29)
A gasoline engine with an efficiency of 40.0% operates between a high temperature T1 and a low temperature T2 = 320 K.If this engine operates with Carnot efficiency,what is the high-side temperature T1?
(Multiple Choice)
4.9/5  (27)
(27)
A turbine takes in 1000 K steam and exhausts the steam at a temperature of 400 K.What is the maximum theoretical efficiency of this system?
(Multiple Choice)
4.8/5  (28)
(28)
Consider a Carnot cycle starting at the state of highest pressure and smallest volume.Call the isothermal expansion that starts at this state step 1,the adiabatic expansion which follows step 2,the isothermal compression that then follows step 3,and the adiabatic compression which returns the system to the initial state step 4.In which step does the entropy of the system increase?
(Multiple Choice)
4.8/5  (29)
(29)
An electrical generating plant operates at a boiler temperature of 220°C and exhausts the unused heat into a nearby river at 19°C.If the generating plant has a power output of 800 megawatts (MW)and if the actual efficiency is 3/5 the theoretical efficiency,how much heat per second must be delivered to the boiler? (0°C = 273 K)
(Multiple Choice)
4.9/5  (36)
(36)
Showing 1 - 20 of 33
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)