Exam 2: Atoms, Elements, and Compounds
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
One of the orbitals in a shell of an atom could be pictured as shown below. 
Free
(True/False)
4.9/5  (30)
(30)
Correct Answer:
True
Which of the following sequences gives the correct order as we move from left to right across a row of the period table?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
A
Write the chemical symbol for the elements that is in period 2 and group 8A of the periodic table.
Free
(Short Answer)
4.9/5  (36)
(36)
Correct Answer:
Ne
Convert the following mass moles.
22.98 g glycine, an amino acid, C2H5NO2.
(Short Answer)
4.9/5  (33)
(33)
Fill in each blank with the appropriate term from the list given below.
protons
neutrons
electrons
valence electrons
-N, P and As have the same number of_____________________.
(Short Answer)
4.9/5  (39)
(39)
Neutral isotopes of the same element have the same number of electrons.
(True/False)
4.7/5  (40)
(40)
Which of the following is the correct unit for the formula weight of a large number of atoms?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following groups of elements contains only metals?
(Multiple Choice)
4.9/5  (37)
(37)
A person drinks 1900 g of water, H2O, per day. How many moles of water did they consume?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following is the conversion factor that could be used to convert a mass in grams of sodium to the corresponding number of moles?
(Multiple Choice)
4.9/5  (34)
(34)
Enter an integer number (1, 2, 3, ...) in the blank.
How many electrons are in shell 2 of a P atom?
____________________electrons
(Short Answer)
4.8/5  (23)
(23)
The pie chart shown below represents the elemental composition of the human body including water. 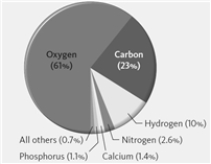
(True/False)
4.8/5  (36)
(36)
Group 4A and Group 14 are two different designations for the same column of the periodic table.
(True/False)
4.8/5  (32)
(32)
A sealed cylinder is filled with a large collection of atoms that have 14 neutrons and 13 protons. Which of the following would behave in a manner similar to this collection of atoms?
(Multiple Choice)
4.9/5  (30)
(30)
Use the following terms as appropriate to complete the given statement. All terms may not be used.
mass
volume
density
intensive
extensive
The ______________________ of a substance is an example of a ____________________ property.
(Short Answer)
4.8/5  (36)
(36)
Complete the following statement using one of the following terms.
compound
element
mixture
In the manufacture of steel, the percent of manganese is adjusted to determine the brittleness of the product. Steel is an example of a ______________________.
(Short Answer)
5.0/5  (34)
(34)
Indicate the number of dots that should be placed around the Lewis symbol for the following element. Use an integer:
1, 2, 3, etc. as appropriate.
Br
(Short Answer)
4.9/5  (44)
(44)
If you need a sample of 2.841 mol of Na2S, how many grams do you need?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)