Exam 5: Solution Concentration
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
-Calculate the parts per million (ppm) of a solution prepared by dissolving 7.45 mg of glucose in enough water to produce 145.0 mL of solution?
Answer the following questions as appropriate with: right,left,or no movement.
-Calculate the parts per million (ppm) of a solution prepared by dissolving 7.45 mg of glucose in enough water to produce 145.0 mL of solution?
Free
(Short Answer)
4.7/5  (38)
(38)
Correct Answer:
51.4 ppm
A solution is prepared by adding 25.0 mL of 1.30 M glucose solution to a flask, and then adding enough water to give a final volume of 200.0 mL. What is the molarity of the solution?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
B
The vitamins A (retinol)and C (ascorbic acid)are shown below.All atoms other than C and H are explicitly shown. 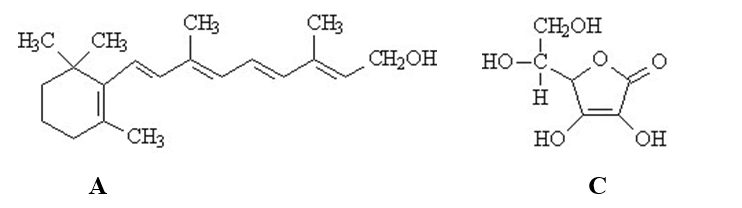 Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The vitamin that would not be stored by the human body is ______.
Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The vitamin that would not be stored by the human body is ______.
Free
(Short Answer)
4.8/5  (40)
(40)
Correct Answer:
C
Consider the solutions shown in the containers below.The composition of each solution is given in the image. 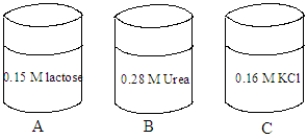 Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
-The tonicity of the solution in container C is ______________________.
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
-The tonicity of the solution in container C is ______________________.
(Short Answer)
4.9/5  (32)
(32)
Saline solutions (NaCl in water) used to deliver intravenous drugs are 0.89%(w/v). What mass of NaCl would be needed to prepare 450.0 mL of such a solution?
(Multiple Choice)
4.9/5  (34)
(34)
The following equation can be used when C represents either a M or % (w/v) concentration. 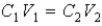
(True/False)
4.9/5  (32)
(32)
Calculate the number of moles of ZnCl2, in 180.0 mL of 0.330 M solution.
(Multiple Choice)
4.9/5  (31)
(31)
The solubility of Na2SO4 will probably increase with increasing temperature.
(True/False)
4.9/5  (36)
(36)
How would the following solution be classified? 0.05 M in KCl and 0.14 M in glucose
(Multiple Choice)
4.8/5  (38)
(38)
How many mL of 6.00 M HCl are needed to prepare 1.50 L of 0.200 M HCl solution?
(Multiple Choice)
4.8/5  (32)
(32)
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
-Upon standing the sucrose molecules will move in which direction?
Answer the following questions as appropriate with: right,left,or no movement.
-Upon standing the sucrose molecules will move in which direction?
(Short Answer)
4.8/5  (35)
(35)
A normal concentration of sodium (Na) in blood plasma is 141 mEq/L (US average range is 135-145 mEq/L). How many mg of sodium are there in a 10.0 mL sample of his blood plasma?
(Multiple Choice)
4.9/5  (42)
(42)
A solution contains 35.5 mg of Vitamin C in 175 mL of solution. The concentration of this solution could be expressed as:
(Multiple Choice)
4.8/5  (38)
(38)
When you have your blood drawn, the most common method of expressing the plasma level of sodium and potassium is as mEq/L.
(True/False)
4.8/5  (29)
(29)
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
-What is the total molarity of a solution containing 25.7 g of sucrose (C12H22O11) and 16.1 g of ribose (C5H10O5) dissolved in enough water to produce 850.0 mL of solution?
Answer the following questions as appropriate with: right,left,or no movement.
-What is the total molarity of a solution containing 25.7 g of sucrose (C12H22O11) and 16.1 g of ribose (C5H10O5) dissolved in enough water to produce 850.0 mL of solution?
(Short Answer)
4.8/5  (40)
(40)
What is the total molarity of solute particles in a 0.250 M solution of (NH4)2SO4?
(Multiple Choice)
4.9/5  (32)
(32)
Calculate the total molarity of solute particles in a solution that contains 0.050 M glucose (a nonelectrolyte) and 0.200 M CaCl2.
(Multiple Choice)
4.8/5  (40)
(40)
The vitamins A (retinol)and C (ascorbic acid)are shown below.All atoms other than C and H are explicitly shown.  Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The vitamin that would be classified as fat-soluble is ______.
Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The vitamin that would be classified as fat-soluble is ______.
(Short Answer)
4.7/5  (29)
(29)
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 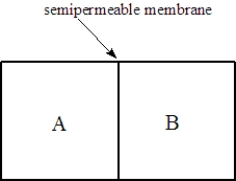 Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.010 M glucose, and solution B is 0.050 M glucose. The glucose will dialyze to the _____________________.
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.010 M glucose, and solution B is 0.050 M glucose. The glucose will dialyze to the _____________________.
(Short Answer)
4.7/5  (37)
(37)
Consider the solutions shown in the containers below.The composition of each solution is given in the image.  Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
-A red blood cell is placed in the solution in container C. The cell will undergo ____________________.
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
-A red blood cell is placed in the solution in container C. The cell will undergo ____________________.
(Short Answer)
4.9/5  (33)
(33)
Showing 1 - 20 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)