Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions
Exam 1: Structure and Bonding30 Questions
Exam 2: Polar Covalent Bonds: Acids and Bases35 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry18 Questions
Exam 5: Stereochemistry at Tetrahedral Centers33 Questions
Exam 6: An Overview of Organic Reactions32 Questions
Exam 7: Alkenes and Alkynes34 Questions
Exam 8: Reactions of Alkenes and Alkynes37 Questions
Exam 9: Aromatic Compounds36 Questions
Exam 10: Structure Determination: Mass Spectrometry,infrared Spectroscopy,and Ultraviolet Spectroscopy41 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy37 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations37 Questions
Exam 13: Alcohols,phenols,and Thiols: Ethers and Sulfides19 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions30 Questions
Exam 15: Carboxylic Acids and Nitriles23 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions42 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions84 Questions
Exam 18: Amines and Heterocycles28 Questions
Exam 19: Biomolecules: Amino Acids,peptides,and Proteins36 Questions
Exam 20: Amino Acid Metabolism34 Questions
Exam 21: Biomolecules: Carbohydrates42 Questions
Exam 22: Carbohydrate Metabolism41 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism31 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism23 Questions
Select questions type
Draw the structure of the diastereomer that is formed preferentially in the following reaction: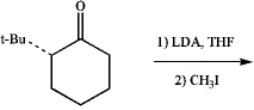
(Essay)
4.8/5  (32)
(32)
Instructions: Consider the reaction below to answer the following question(s). 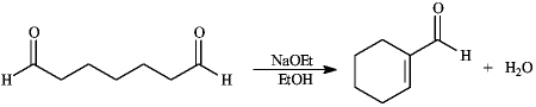 Refer to instructions.The product of this reaction is:
Refer to instructions.The product of this reaction is:
(Multiple Choice)
5.0/5  (42)
(42)
Which of the following are intermediates in the acid catalyzed aldol reaction of propanal to form 2-methyl-pent-2-enal? 
(Multiple Choice)
4.9/5  (38)
(38)
Instructions: Consider the reaction below to answer the following question(s).
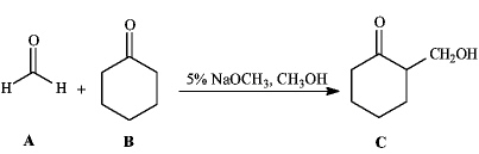 -Refer to instructions.This reaction is an example of:
-Refer to instructions.This reaction is an example of:
(Multiple Choice)
4.8/5  (40)
(40)
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s).If a compound does not undergo aldol self-condensation,explain why it does not.
-Draw and explain: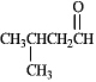
(Essay)
4.9/5  (39)
(39)
How would you prepare 3-phenylpropanoic acid using a malonic ester synthesis?
(Essay)
4.8/5  (36)
(36)
Instructions: Consider the reaction sequence below to answer the following question(s). 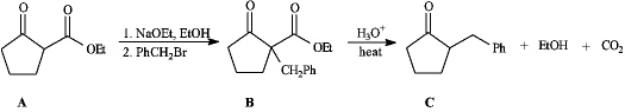 Refer to instructions.Conversion of A into B is a type of reaction termed _____.
Refer to instructions.Conversion of A into B is a type of reaction termed _____.
(Multiple Choice)
4.8/5  (35)
(35)
Rank the following hydrogens in terms of decreasing acidity (most acidic > least acidic):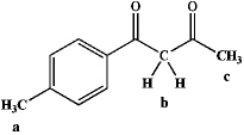
(Essay)
4.9/5  (38)
(38)
Identify the intermediate imine that results from the following reaction.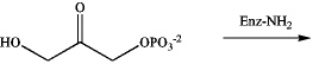
(Essay)
4.8/5  (31)
(31)
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s).If a compound does not undergo aldol self-condensation,explain why it does not.
-Draw and explain: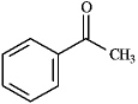
(Essay)
4.8/5  (32)
(32)
Instructions: Draw the structures of the precursors to the Michael reaction products shown in the question(s)below.Label the Michael donor and the Michael acceptor in each case and formulate the reaction.
-Draw and label: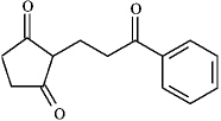
(Essay)
4.8/5  (33)
(33)
Rank the following hydrogens in terms of decreasing acidity (most acidic > least acidic):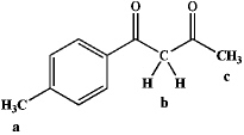
(Essay)
4.9/5  (32)
(32)
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s).If a compound does not undergo aldol self-condensation,explain why it does not.
-Draw and explain: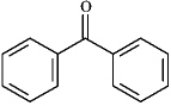
(Essay)
5.0/5  (40)
(40)
Instructions: Consider the reaction sequence below to answer the following question(s).
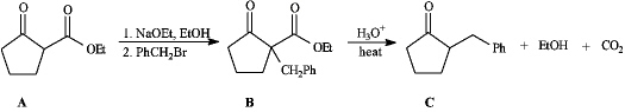 -Refer to instructions.Conversion of B into C involves hydrolysis of the ester followed by decarboxylation.On the structures provided below,show the electron flow for the decarboxylation step.
-Refer to instructions.Conversion of B into C involves hydrolysis of the ester followed by decarboxylation.On the structures provided below,show the electron flow for the decarboxylation step.
(Essay)
4.8/5  (33)
(33)
Draw the structure of the diastereomer that is formed preferentially in the following reaction: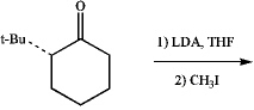
(Essay)
4.8/5  (33)
(33)
Instructions: Consider the reaction below to answer the following question(s). 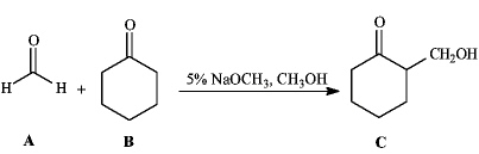 Refer to instructions.This reaction is an example of:
Refer to instructions.This reaction is an example of:
(Multiple Choice)
4.7/5  (29)
(29)
Which of the following is common to both tautomers and resonance forms of a compound?
(Multiple Choice)
4.8/5  (39)
(39)
Instructions: Each of the following compounds in the following question(s)can be prepared by a mixed aldol condensation reaction.
a)Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.
b)Give the structure of the intermediate aldol product.
-Refer to instructions.Use the following compound: 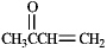
(Essay)
4.9/5  (43)
(43)
Instructions: Consider the reaction below to answer the following question(s).
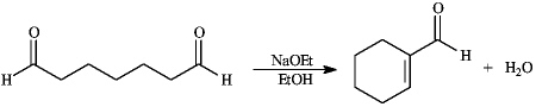 -Refer to instructions.This reaction is an example of:
-Refer to instructions.This reaction is an example of:
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following are intermediates in the acid catalyzed aldol reaction of propanal to form 2-methyl-pent-2-enal?

(Multiple Choice)
4.9/5  (28)
(28)
Showing 21 - 40 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)