Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions
Exam 1: Structure and Bonding30 Questions
Exam 2: Polar Covalent Bonds: Acids and Bases35 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry18 Questions
Exam 5: Stereochemistry at Tetrahedral Centers33 Questions
Exam 6: An Overview of Organic Reactions32 Questions
Exam 7: Alkenes and Alkynes34 Questions
Exam 8: Reactions of Alkenes and Alkynes37 Questions
Exam 9: Aromatic Compounds36 Questions
Exam 10: Structure Determination: Mass Spectrometry,infrared Spectroscopy,and Ultraviolet Spectroscopy41 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy37 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations37 Questions
Exam 13: Alcohols,phenols,and Thiols: Ethers and Sulfides19 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions30 Questions
Exam 15: Carboxylic Acids and Nitriles23 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions42 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions84 Questions
Exam 18: Amines and Heterocycles28 Questions
Exam 19: Biomolecules: Amino Acids,peptides,and Proteins36 Questions
Exam 20: Amino Acid Metabolism34 Questions
Exam 21: Biomolecules: Carbohydrates42 Questions
Exam 22: Carbohydrate Metabolism41 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism31 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism23 Questions
Select questions type
Instructions: Consider the structures below to answer the following question(s). 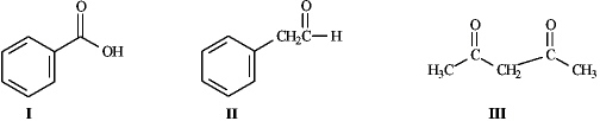 Refer to instructions.Rank the molecules above in order of increasing acidity (least acidic to most acidic).
Refer to instructions.Rank the molecules above in order of increasing acidity (least acidic to most acidic).
(Multiple Choice)
4.7/5  (33)
(33)
Instructions: Consider the reaction sequence below to answer the following question(s). 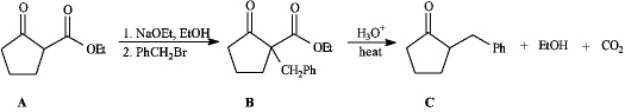 Refer to instructions.The starting material A in this reaction sequence is called a(n)_____.
Refer to instructions.The starting material A in this reaction sequence is called a(n)_____.
(Multiple Choice)
4.9/5  (36)
(36)
When treated with base and heat,the following diketone undergoes an intramolecular aldol reaction followed by dehydration to produce cis-jasmone,a perfume component.Draw the structure of the product.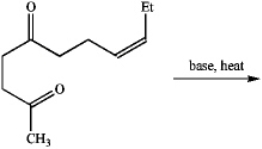
(Essay)
4.9/5  (38)
(38)
Instructions: Give the major organic product(s)of each reaction or sequences of reactions for the following question(s).Show all relevant stereochemistry.
-Give major product(s):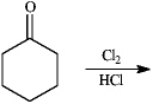
(Essay)
4.7/5  (46)
(46)
Instructions: Consider the structures below to answer the following question(s). 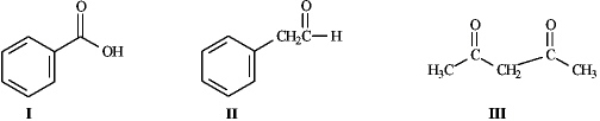 -Refer to instructions.Underline the acidic hydrogen atoms in each of the molecules.
-Refer to instructions.Underline the acidic hydrogen atoms in each of the molecules.
(Essay)
4.7/5  (33)
(33)
Instructions: The Stork enamine reaction is a variation on the Michael reaction that utilizes an enamine nucleophile.Use this information to answer the following question.
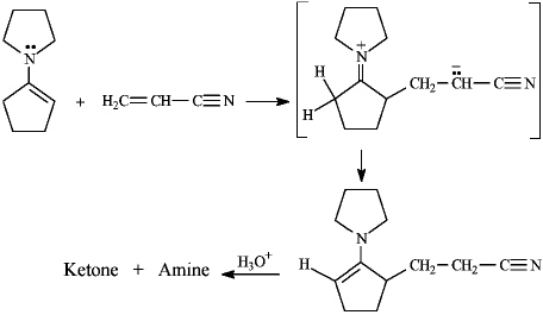 -Refer to instructions.Draw the structures of the ketone and amine products of this reaction.
-Refer to instructions.Draw the structures of the ketone and amine products of this reaction.
(Essay)
5.0/5  (36)
(36)
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s).If a compound does not undergo aldol self-condensation,explain why it does not.
-Draw and explain: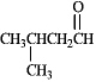
(Essay)
4.8/5  (47)
(47)
Instructions: Consider the reaction below to answer the following question(s).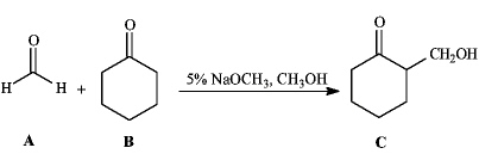 -Refer to instructions.Which carbonyl compound functions as the electrophile in this reaction?
-Refer to instructions.Which carbonyl compound functions as the electrophile in this reaction?
(Multiple Choice)
4.9/5  (34)
(34)
Instructions: Draw the structure of the product you would expect to obtain by Claisen condensation of the esters shown in the question(s)below.If an ester does not undergo Claisen condensation,explain why it does not.
-Draw and explain: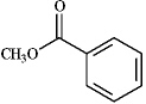
(Essay)
4.8/5  (43)
(43)
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s).If a compound does not undergo aldol self-condensation,explain why it does not.
-Draw and explain: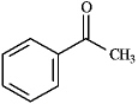
(Essay)
4.9/5  (40)
(40)
Instructions: Refer to the compounds below to answer the following question(s).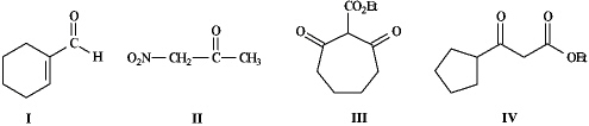 - Refer to instructions.Underline all the acidic hydrogen atoms in Compounds I through IV.
- Refer to instructions.Underline all the acidic hydrogen atoms in Compounds I through IV.
(Essay)
4.7/5  (47)
(47)
Instructions: Consider the reaction below to answer the following question(s).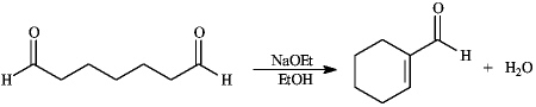 -Refer to instructions.Write the complete stepwise mechanism for the reaction above.Show all intermediate structures and all electron flow with arrows.
-Refer to instructions.Write the complete stepwise mechanism for the reaction above.Show all intermediate structures and all electron flow with arrows.
(Essay)
4.7/5  (33)
(33)
How would you prepare 5-methyl-2-hexanone using an acetoacetic ester synthesis?
(Essay)
4.8/5  (36)
(36)
Instructions: Consider the structures below to answer the following question(s).
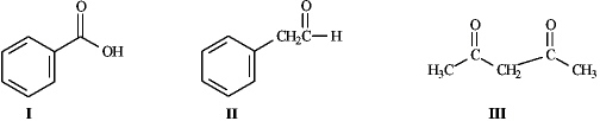 -Refer to instructions.Rank the molecules above in order of increasing acidity (least acidic to most acidic).
-Refer to instructions.Rank the molecules above in order of increasing acidity (least acidic to most acidic).
(Multiple Choice)
4.7/5  (29)
(29)
Which of the following would form an enolate ion on treatment with a base? 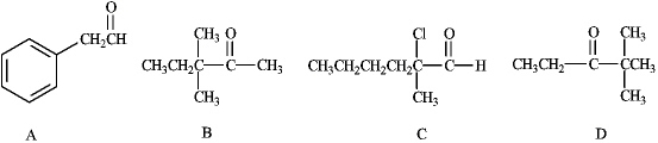
(Multiple Choice)
4.7/5  (36)
(36)
Instructions: Draw the structures of the precursors to the Michael reaction products shown in the question(s)below.Label the Michael donor and the Michael acceptor in each case and formulate the reaction.
-Draw and label: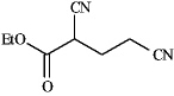
(Essay)
4.8/5  (42)
(42)
Instructions: Give the major organic product(s)of each reaction or sequences of reactions for the following question(s).Show all relevant stereochemistry.
Give major product(s): 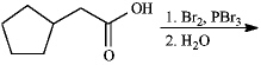
(Essay)
4.8/5  (34)
(34)
Showing 41 - 60 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)