Exam 1: Structure and Bonding
Exam 1: Structure and Bonding30 Questions
Exam 2: Polar Covalent Bonds: Acids and Bases35 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry18 Questions
Exam 5: Stereochemistry at Tetrahedral Centers33 Questions
Exam 6: An Overview of Organic Reactions32 Questions
Exam 7: Alkenes and Alkynes34 Questions
Exam 8: Reactions of Alkenes and Alkynes37 Questions
Exam 9: Aromatic Compounds36 Questions
Exam 10: Structure Determination: Mass Spectrometry,infrared Spectroscopy,and Ultraviolet Spectroscopy41 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy37 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations37 Questions
Exam 13: Alcohols,phenols,and Thiols: Ethers and Sulfides19 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions30 Questions
Exam 15: Carboxylic Acids and Nitriles23 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions42 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions84 Questions
Exam 18: Amines and Heterocycles28 Questions
Exam 19: Biomolecules: Amino Acids,peptides,and Proteins36 Questions
Exam 20: Amino Acid Metabolism34 Questions
Exam 21: Biomolecules: Carbohydrates42 Questions
Exam 22: Carbohydrate Metabolism41 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism31 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism23 Questions
Select questions type
Specify the hybridization of each carbon atom of limonene,a natural product present in citrus fruits,and thujone,which is derived from wormwood,a traditional component of the notorious liquor,Absinthe.
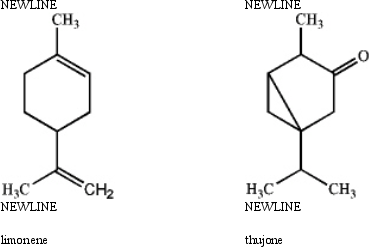
(Essay)
4.9/5  (37)
(37)
Draw all possible structures of CFnClm where n and m vary from 0 to 4.
(Essay)
4.8/5  (35)
(35)
Which of the following statements is not true according to molecular orbital (MO)theory?
(Multiple Choice)
4.9/5  (42)
(42)
Draw all the lone pairs (nonbonding valence electrons)on the structure of phosgene,a poisonous gas once used as a chemical warfare agent.
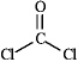
(Essay)
4.7/5  (34)
(34)
Instructions: Propose a structure for a molecule that meets the following description.
Refer to instructions.Contains only two sp3 hybridized carbons and two sp hybridized carbons.
(Essay)
4.8/5  (38)
(38)
Instructions: Determine the hybridization for the indicated atoms in each structure below.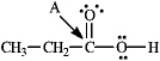
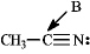 Refer to instructions.The hybridization of carbon atom A is _____.
Refer to instructions.The hybridization of carbon atom A is _____.
(Short Answer)
4.7/5  (30)
(30)
Instructions: Determine the hybridization for the indicated atoms in each structure below.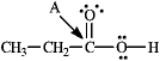
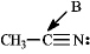 Refer to instructions.The hybridization of carbon atom B is _____.
Refer to instructions.The hybridization of carbon atom B is _____.
(Short Answer)
4.9/5  (40)
(40)
Which of the following best represents the shape of a 2p atomic orbital of carbon? 
(Multiple Choice)
5.0/5  (36)
(36)
Convert the skeletal drawing of the pharmaceutical Vioxx into a molecular formula.
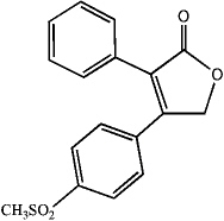
(Short Answer)
4.8/5  (29)
(29)
Showing 21 - 30 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)