Exam 7: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
A ground-state atom of manganese has ___ unpaired electrons and is _____.
(Multiple Choice)
4.9/5  (44)
(44)
List the following sets of quantum numbers in order of increasing energy: I.n = 4, l = 0, ml = 0, ms = -1/2
II.n = 4, l = 2, ml = -1, ms = -1/2
III.n = 5, l = 0, ml = 0, ms = +1/2
(Multiple Choice)
4.9/5  (41)
(41)
Calculate the energy of a photon of light with a wavelength of 360 nm.
(Short Answer)
5.0/5  (37)
(37)
An AM radio station broadcasts at a frequency of 1270 kHz.Calculate the wavelength of the broadcast signal in meters.(c = 2.9979 × 108 m/s)
(Short Answer)
4.8/5  (34)
(34)
If a hydrogen atom and a helium atom are traveling at the same speed,
(Multiple Choice)
4.7/5  (39)
(39)
If one electron is added to the outer shell of chlorine, it would have the same electron configuration as what element?
(Short Answer)
4.8/5  (36)
(36)
What is the maximum number of electrons in an atom that can have the following quantum numbers? n = 3 l = 2
(Multiple Choice)
4.7/5  (36)
(36)
A possible set of quantum numbers for the last electron added to complete an atom of germanium (Ge)in its ground state is 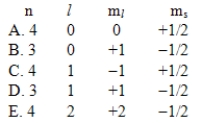
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following is the electron configuration of an excited state of a copper atom?
(Multiple Choice)
4.7/5  (39)
(39)
The ground-state electron configuration for an atom of indium is
(Multiple Choice)
4.9/5  (43)
(43)
Calculate the energy, in joules, required to excite a hydrogen atom by causing an electronic transition from the n = 1 to the n = 4 principal energy level.Recall that the energy levels of the H atom are given by En = -2.18 × 10-18 J(1/n2)
(Multiple Choice)
4.8/5  (36)
(36)
A possible set of quantum numbers to describe an electron in a 4s subshell is
(Multiple Choice)
4.9/5  (29)
(29)
What is the wavelength, in meters, of an alpha particle with a kinetic energy of 8.0 × 10-13 J.(mass of an alpha particle = 4.00150 amu; 1 amu = 1.67 × 10-27 kg)
(Short Answer)
4.9/5  (38)
(38)
Which one of the following sets of quantum numbers represents an electron with the highest energy?
(Multiple Choice)
4.9/5  (39)
(39)
A ground-state atom of vanadium has ___ unpaired electrons and is _____.
(Multiple Choice)
4.9/5  (36)
(36)
The colors of the visible spectrum are blue, green, orange, red, violet, and yellow.Of these colors, ______ has the least energy.
(Short Answer)
4.8/5  (36)
(36)
When the electron in a hydrogen atom falls from the n = 3 excited energy level to the ground state energy level, a photon with wavelength is emitted.An electron having this same wavelength would have a velocity of
(Multiple Choice)
4.9/5  (43)
(43)
What is the maximum number of electrons in an atom that can have the following set of quantum numbers? n = 4 l = 3 ml = -2 ms = +1/2
(Multiple Choice)
4.8/5  (35)
(35)
Showing 41 - 60 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)