Exam 7: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
The quantum numbers, n = 3, l = 1, ml = 0, ms = +1/2, represent an electron in a 3s subshell.
(True/False)
4.8/5  (42)
(42)
The frequency of the emitted light from a cesium atom is an intensive property.
(True/False)
4.8/5  (42)
(42)
Which one of the following sets of quantum numbers is not possible? 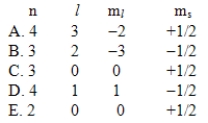
(Multiple Choice)
4.8/5  (33)
(33)
What is the wavelength of radiation that has a frequency of 3.4 x 1011 s -1?
(Multiple Choice)
4.8/5  (40)
(40)
Calculate the wavelength of a neutron that has a velocity of 250 cm/s.(The mass of a neutron = 1.675 × 10-24 g)
(Multiple Choice)
4.8/5  (42)
(42)
In an electron microscope, electrons are accelerated to great velocities.Calculate the wavelength of an electron traveling with a velocity of 7.0 × 103 kilometers per second.The mass of an electron is 9.1 × 10-28 g.
(Multiple Choice)
4.9/5  (36)
(36)
Which ground-state atom has an electron configuration described by the following orbital diagram? 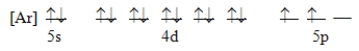
(Multiple Choice)
5.0/5  (38)
(38)
When photons with a wavelength of 310.nm strike a magnesium plate, the maximum velocity of the ejected electrons is 3.45 × 105 m/s.Calculate the binding energy of electrons to the magnesium surface.
(Multiple Choice)
4.8/5  (37)
(37)
The scattering of helium atoms by a solid can be used to gain information about the lattice of atoms, provided the wavelength of the helium atoms is comparable to the dimensions of the lattice.What is the wavelength, in picometers, of a helium-4 atom with a kinetic energy of 0.10 eV.(mass of 4He = 4.0026 amu; 1 amu = 1.67 × 10-24 g; 1 eV = 1.602 × 10-19 J. h = 6.626 × 10-34 J.s)
(Short Answer)
4.9/5  (33)
(33)
What is the wavelength of radiation that has a frequency of 5.39 × 1014 s-1? (c = 2.9979 × 108 m/s)
(Multiple Choice)
4.9/5  (33)
(33)
Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level.Recall that for hydrogen En = -2.18 × 10 -18 J(1/n2)
(Multiple Choice)
4.7/5  (36)
(36)
Using the figure below, categorize electromagnetic radiation with an energy of 6.6 x 10-16 J/photon. 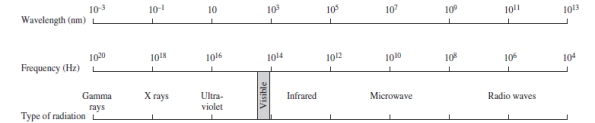
(Multiple Choice)
4.9/5  (34)
(34)
Electrons can be used to probe the arrangement of atoms on a solid surface if the wavelength of the electrons is comparable with the spacing between the atoms.Which of the following electron velocities would be appropriate for use in this application if the atoms are separated by 0.320 nm?
(Multiple Choice)
4.8/5  (38)
(38)
Which element has the following ground-state electron configuration? 1s22s22p63s2
(Multiple Choice)
4.9/5  (45)
(45)
The quantum numbers, n = 4, l = 3, ml = 2, ms = +1/2, represent an electron in a 4f subshell
(True/False)
4.7/5  (38)
(38)
The electron in a hydrogen atom falls from an excited energy level to the ground state in two steps, causing the emission of photons with wavelengths of 2624 nm and 97.2 nm, respectively.What is the principal quantum number or shell of the initial excited energy level from which the electron falls?
(Multiple Choice)
4.9/5  (27)
(27)
Which one of the following sets of quantum numbers is not possible? 
(Multiple Choice)
4.8/5  (33)
(33)
How many unpaired electrons does an atom of sulfur have in its ground state?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 61 - 80 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)