Exam 7: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
The second line of the Balmer series occurs at a wavelength of 486.1 nm.What is the energy difference between the initial and final levels of the hydrogen atom in this emission process?
(Multiple Choice)
4.8/5  (43)
(43)
The following set of quantum numbers is correct.
n = 3, l = 3, ml = 0, ms = +1/2
(True/False)
4.9/5  (41)
(41)
Rank the following types of electromagnetic radiation from lowest energy to highest energy: infrared, microwave, radio waves, gamma rays, visible, and ultraviolet.
(Essay)
4.8/5  (32)
(32)
Write the ground state electron configuration for the phosphorus atom.
(Essay)
4.8/5  (31)
(31)
Which element has the following ground-state electron configuration? [Kr]5s24d105p3
(Multiple Choice)
4.9/5  (50)
(50)
There is nothing wrong with the following set of quantum numbers:
n = 3, l = 1, ml = 0, ms = +1/2
(True/False)
4.8/5  (42)
(42)
How many unpaired electrons does an atom of carbon have in its ground state?
(Multiple Choice)
5.0/5  (43)
(43)
Breaking the oxygen-oxygen bond in hydrogen peroxide requires 210 kJ/mol.What is the longest wavelength of light that can cause this bond to be broken?
(Multiple Choice)
4.8/5  (28)
(28)
The electron configuration of a ground-state copper atom is
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following atoms is diamagnetic in its ground-state?
(Multiple Choice)
4.8/5  (39)
(39)
Each shell (principal energy level)of quantum number n contains n subshells
(True/False)
4.8/5  (39)
(39)
A photon is roughly 1800 times more massive than an electron.If a proton and an electron have the same kinetic energy,
(Multiple Choice)
4.9/5  (36)
(36)
If we take away two electrons from the outer shell of calcium, it would have the same electron configuration as what element?
(Short Answer)
4.8/5  (31)
(31)
What is the wavelength (nm)for a photon of light with an energy of 3.20 x 10-19 J/photon?
(Short Answer)
4.8/5  (42)
(42)
What is the energy in joules of a mole of photons associated with red light of wavelength 7.00 × 102 nm?
(Multiple Choice)
4.9/5  (37)
(37)
Using the figure below, categorize electromagnetic radiation with an energy of 6.7 x 10-18 J/photon. 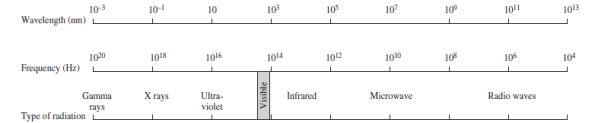
(Multiple Choice)
4.8/5  (43)
(43)
Showing 81 - 100 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)