Exam 7: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
A photovoltaic cell converts light into electrical energy.Suppose a certain photovoltaic cell is only 63.5% efficient, in other words, that 63.5% of the light energy is ultimately recovered.If the energy output of this cell is used to heat water, how many 520 nm photons must be absorbed by the photovoltaic cell in order to heat 10.0 g of water from 20.0°C to 30.0°?
(Short Answer)
4.7/5  (38)
(38)
Which ground-state atom has an electron configuration described by the following orbital diagram? 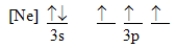
(Multiple Choice)
4.8/5  (37)
(37)
Which element has the following ground-state electron configuration? [Xe]6s24f145d4
(Multiple Choice)
4.7/5  (36)
(36)
"No two electrons in an atom can have the same four quantum numbers" is a statement of
(Multiple Choice)
4.8/5  (42)
(42)
An FM radio station broadcasts at a frequency of 101.7 MHz.Calculate the wavelength of the broadcast signal in meters.(c = 2.9979 × 108 m/s)
(Short Answer)
4.7/5  (40)
(40)
The quantum numbers, n = 4, l = 1, ml = 1, ms = +1/2, represent an electron is a 4p subshell.
(True/False)
4.8/5  (30)
(30)
What is the total number of electrons possible in the 6s orbital?
(Short Answer)
4.9/5  (46)
(46)
The colors of the visible spectrum are blue, green, orange, red, violet, and yellow.Of these colors, _______ has the longest wavelength.
(Short Answer)
4.9/5  (36)
(36)
The ground-state electron configuration of Cr, Mo, and Ag are exceptions to the Aufbau principle.Which of the following is the electron configuration for Mo?
(Multiple Choice)
4.8/5  (38)
(38)
Which element has the following ground-state electron configuration? [Ar]4s23d104p5
(Multiple Choice)
4.9/5  (44)
(44)
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
(Multiple Choice)
4.9/5  (39)
(39)
What is the maximum number of electrons in an atom that can have the following set of quantum numbers? n = 3 l = 2 ml = -2
(Multiple Choice)
4.9/5  (29)
(29)
Showing 121 - 136 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)