Exam 1: Introduction: Matter and Measurement
Exam 1: Introduction: Matter and Measurement151 Questions
Exam 2: Atoms, Molecules, and Ions230 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations170 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry177 Questions
Exam 5: Thermochemistry148 Questions
Exam 6: Electronic Structure of Atoms180 Questions
Exam 7: Periodic Properties of the Elements171 Questions
Exam 8: Basic Concepts of Chemical Bonding141 Questions
Exam 9: Molecular Geometry and Bonding Theories177 Questions
Exam 10: Gases172 Questions
Exam 11: Liquids and Intermolecular Forces119 Questions
Exam 12: Solids and Modern Materials78 Questions
Exam 13: Properties of Solutions151 Questions
Exam 14: Chemical Kinetics130 Questions
Exam 15: Chemical Equilibrium92 Questions
Exam 16: Acid-Base Equilibria134 Questions
Exam 17: Additional Aspects of Aqueous Equilibria111 Questions
Exam 18: Chemistry of the Environment121 Questions
Exam 19: Chemical Thermodynamics120 Questions
Exam 20: Electrochemistry110 Questions
Exam 21: Nuclear Chemistry158 Questions
Exam 22: Chemistry of the Nonmetals192 Questions
Exam 23: Transition Metals and Coordination Chemistry147 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry124 Questions
Select questions type
One edge of a cube is measured and found to be 13 cm. The volume of the cube in m3 is __________.
(Multiple Choice)
4.8/5  (30)
(30)
A 4.369 g sample of metal is placed in a flask. Water is added to the flask and the total volume in the flask is read to be 126.4 ml. The mass of the water, flask, and metal is 268.5 g. If the mass of the flask is 139.3 g and the density of water is 1.000 g/ml, the density of the solid is __________ g/cm3.
(Multiple Choice)
4.8/5  (46)
(46)
How many liters of wine can be held in a wine barrel whose capacity is 26.0 gal? 1 gal = 4 qt = 3,7854 L.
(Multiple Choice)
4.9/5  (37)
(37)
A small amount of salt dissolved in water is an example of a __________.
(Multiple Choice)
4.8/5  (41)
(41)
The quantity 1.0 mg/cm2 is the same as 1.0 × __________ kg/m2.
(Multiple Choice)
4.8/5  (40)
(40)
An iron mine produces 1.67 x 104 tons of raw ore per day. If the ore is 26.39% elemental iron, the mine produces __________ pounds of elemental iron per year. (Assume the mine operates 365 days per year.)
(Multiple Choice)
4.8/5  (36)
(36)
A cube of an unknown metal measures 1.61 mm on one side. The mass of the cube is 36 mg. Which of the following is most likely the unknown metal? 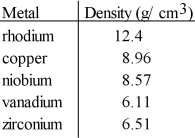
(Multiple Choice)
4.9/5  (26)
(26)
The correct result of the molecular mass calculation for H2SO4 is ________. 4 × 15.9994 + 32.066 + 2 × 1.0079 =
(Multiple Choice)
4.8/5  (37)
(37)
A certain liquid has a density of 2.67 g/cm3. 30.5 mL of this liquid would have a mass of __________ Kg.
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the following has the element name and symbol correctly matched?
(Multiple Choice)
4.7/5  (34)
(34)
Showing 41 - 60 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)