Exam 1: Introduction: Matter and Measurement
Exam 1: Introduction: Matter and Measurement151 Questions
Exam 2: Atoms, Molecules, and Ions230 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations170 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry177 Questions
Exam 5: Thermochemistry148 Questions
Exam 6: Electronic Structure of Atoms180 Questions
Exam 7: Periodic Properties of the Elements171 Questions
Exam 8: Basic Concepts of Chemical Bonding141 Questions
Exam 9: Molecular Geometry and Bonding Theories177 Questions
Exam 10: Gases172 Questions
Exam 11: Liquids and Intermolecular Forces119 Questions
Exam 12: Solids and Modern Materials78 Questions
Exam 13: Properties of Solutions151 Questions
Exam 14: Chemical Kinetics130 Questions
Exam 15: Chemical Equilibrium92 Questions
Exam 16: Acid-Base Equilibria134 Questions
Exam 17: Additional Aspects of Aqueous Equilibria111 Questions
Exam 18: Chemistry of the Environment121 Questions
Exam 19: Chemical Thermodynamics120 Questions
Exam 20: Electrochemistry110 Questions
Exam 21: Nuclear Chemistry158 Questions
Exam 22: Chemistry of the Nonmetals192 Questions
Exam 23: Transition Metals and Coordination Chemistry147 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry124 Questions
Select questions type
There are __________ significant figures in the answer to the following computation: 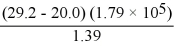
(Multiple Choice)
4.7/5  (34)
(34)
Which one of the following has the element name and symbol correctly matched?
(Multiple Choice)
4.7/5  (29)
(29)
You have to calculate the mass of a 30.0 mL liquid sample with density of 1.52 g/mL, but you have forgotten the formula. Which way of reasoning would help you in finding the correct mass?
(Multiple Choice)
4.9/5  (35)
(35)
One angstrom, symbolized Å, is 10-10 m. 1 cm3 = __________ Å3
(Multiple Choice)
4.8/5  (33)
(33)
The correct result (indicating the proper number of significant figures)of the following addition is __________. 12
1.2
0.12
+ 0.012
(Multiple Choice)
4.9/5  (37)
(37)
One side of a cube measures 1.55 m. The volume of this cube is __________ 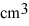 .
.
(Multiple Choice)
5.0/5  (23)
(23)
__________ significant figures should be retained in the result of the following calculation. 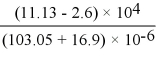
(Multiple Choice)
4.9/5  (31)
(31)
Water is considered to be a diatomic molecule because it is composed of two different atoms.
(True/False)
4.9/5  (29)
(29)
Showing 21 - 40 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)