Exam 8: Electron Delocalization, Resonance, and Aromaticity More About Molecular Orbital Theory
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding81 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry37 Questions
Exam 3: An Introduction to Organic Compounds123 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space111 Questions
Exam 5: Alkenes Thermodynamics and Kinetics76 Questions
Exam 6: Reactions of Alkenes93 Questions
Exam 7: Reactions of Alkynes Introduction to Multistep Synthesis119 Questions
Exam 8: Electron Delocalization, Resonance, and Aromaticity More About Molecular Orbital Theory166 Questions
Exam 9: Substitution Reactions of Alkyl Halides118 Questions
Exam 10: Elimination Reactions of Alkyl Halides Competition Between Substitution and Elimination94 Questions
Exam 11: Reactions of Alcohols, Ethers, Epoxides, and Sulfur-Containing Compounds100 Questions
Exam 12: Organometallic Compounds57 Questions
Exam 13: Reactions of Alkanes, Radicals130 Questions
Exam 14: Mass Spectrometry, Infrared Spectroscopy, and Uvvis Spectroscopy127 Questions
Exam 15: Nmr Spectroscopy110 Questions
Exam 16: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives128 Questions
Exam 17: Reactions of Aldehydes and Ketones116 Questions
Exam 18: Reactions at the Alpha Carbon of Carbonyl Compounds113 Questions
Exam 19: Reactions of Benzene and Substituted Benzenes155 Questions
Exam 20: More About Amines Heterocylic Compounds115 Questions
Exam 21: Carbohydrates100 Questions
Exam 22: Amino Acids, Peptides, and Proteins105 Questions
Exam 23: Catalysis85 Questions
Exam 24: The Organic Mechanisms of the Coenzymes92 Questions
Exam 25: The Chemistry of Metabolism94 Questions
Exam 26: Nucleosides, Nucleotides, and Nucleic Acids85 Questions
Exam 27: Synthetic Polymers106 Questions
Exam 28: Pericyclic Reactions92 Questions
Select questions type
Which of the following statements is (are)true of the Diels-Alder reaction?
(Multiple Choice)
4.8/5  (38)
(38)
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 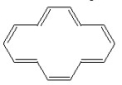
(Short Answer)
4.9/5  (33)
(33)
Which of the following is/are the major product(s)of the following reaction? 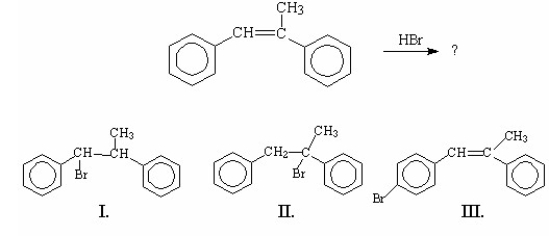
(Multiple Choice)
4.9/5  (40)
(40)
Indicate the compound that has the shortest bond length between the two middle carbon atoms.
(Multiple Choice)
4.9/5  (37)
(37)
When cycloheptatriene is deprotonated, an anion with seven resonance forms of equal energy can be drawn. Given this fact, explain why cycloheptatriene is only slightly more acidic than propene.
(Essay)
4.8/5  (37)
(37)
Which of the following statements about the π molecular orbital description of cyclobutadiene is not correct?
(Multiple Choice)
4.7/5  (38)
(38)
Give the hybridization, shape, and bond angle of a carbon in benzene.
(Short Answer)
4.7/5  (35)
(35)
Draw four resonance contributors for the following structure and indicate which is the most important contributor. Explain. 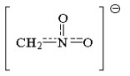
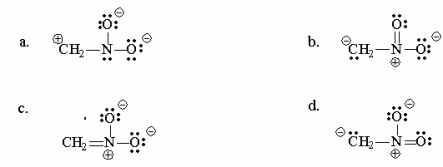
(Essay)
4.8/5  (46)
(46)
Draw the important resonance contributing forms for the structure shown below. 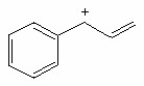
(Essay)
5.0/5  (37)
(37)
Cyclic hydrocarbons which can be represented as structures containing alternating single and double bonds are called ________.
(Short Answer)
4.9/5  (38)
(38)
Provide the structure of the major organic product in the following reaction. 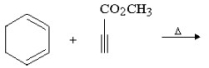
(Essay)
4.8/5  (37)
(37)
Arrange the following unsaturated hydrocarbons in order of decreasing reactivity toward HBr addition: 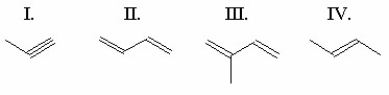
(Short Answer)
4.9/5  (35)
(35)
Draw the distribution of π electrons in the molecular orbitals of cyclobutadiene. 
(Essay)
4.7/5  (33)
(33)
Showing 41 - 60 of 166
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)