Exam 8: Electron Delocalization, Resonance, and Aromaticity More About Molecular Orbital Theory
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding81 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry37 Questions
Exam 3: An Introduction to Organic Compounds123 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space111 Questions
Exam 5: Alkenes Thermodynamics and Kinetics76 Questions
Exam 6: Reactions of Alkenes93 Questions
Exam 7: Reactions of Alkynes Introduction to Multistep Synthesis119 Questions
Exam 8: Electron Delocalization, Resonance, and Aromaticity More About Molecular Orbital Theory166 Questions
Exam 9: Substitution Reactions of Alkyl Halides118 Questions
Exam 10: Elimination Reactions of Alkyl Halides Competition Between Substitution and Elimination94 Questions
Exam 11: Reactions of Alcohols, Ethers, Epoxides, and Sulfur-Containing Compounds100 Questions
Exam 12: Organometallic Compounds57 Questions
Exam 13: Reactions of Alkanes, Radicals130 Questions
Exam 14: Mass Spectrometry, Infrared Spectroscopy, and Uvvis Spectroscopy127 Questions
Exam 15: Nmr Spectroscopy110 Questions
Exam 16: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives128 Questions
Exam 17: Reactions of Aldehydes and Ketones116 Questions
Exam 18: Reactions at the Alpha Carbon of Carbonyl Compounds113 Questions
Exam 19: Reactions of Benzene and Substituted Benzenes155 Questions
Exam 20: More About Amines Heterocylic Compounds115 Questions
Exam 21: Carbohydrates100 Questions
Exam 22: Amino Acids, Peptides, and Proteins105 Questions
Exam 23: Catalysis85 Questions
Exam 24: The Organic Mechanisms of the Coenzymes92 Questions
Exam 25: The Chemistry of Metabolism94 Questions
Exam 26: Nucleosides, Nucleotides, and Nucleic Acids85 Questions
Exam 27: Synthetic Polymers106 Questions
Exam 28: Pericyclic Reactions92 Questions
Select questions type
How could the following compound be synthesized using a Diels-Alder reaction? 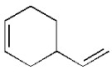
(Essay)
4.8/5  (42)
(42)
Provide the structure of the major organic product in the following reaction. 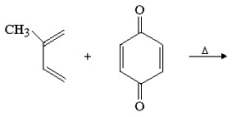
(Essay)
4.7/5  (38)
(38)
Classify cyclopheptatrienyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.
(Short Answer)
4.7/5  (43)
(43)
Which of the following dienes is the most reactive in a Diels-Alder reaction? 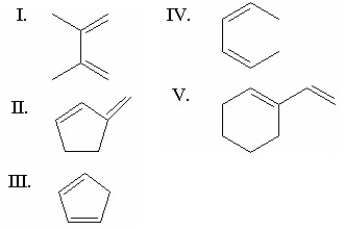
(Multiple Choice)
4.9/5  (37)
(37)
Provide the structure of the major organic product in the following reaction. 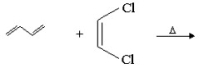
(Essay)
4.9/5  (44)
(44)
Which of the following numbered hydrogens is most easily abstracted by heterolytic cleavage? Explain. 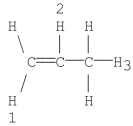
(Essay)
4.9/5  (33)
(33)
Which species is represented by the following distribution of p electrons in the molecular energy diagram? 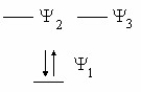
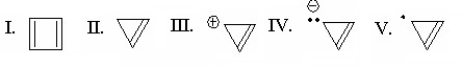
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following statements concerning resonance contributors and resonance hybrids is not correct?
(Multiple Choice)
4.9/5  (39)
(39)
Rank the following dienes in order of increasing stability: trans-1,3-pentadiene, cis-1,3-pentadiene, 1,4-pentadiene, and 1,2-pentadiene.
(Essay)
4.9/5  (33)
(33)
The delocalized π system in benzene is formed by a cyclic overlap of 6 ________ orbitals.
(Multiple Choice)
4.8/5  (40)
(40)
Consider the hydrogenation reaction of each compound listed and rank the compounds in order of increasing ΔH° of this reaction. The most negative ΔH° should be listed first.
cis-2-pentene, 2,3-pentadiene, and trans-1,3-pentadiene
(Essay)
4.7/5  (36)
(36)
Showing 101 - 120 of 166
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)