Exam 7: Covalent Bonding and Electron-Dot Structures
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Among the compounds H3C-CH3,H2C=CH2,and HC  CH,the compound with the strongest carbon-carbon bond is ________,and the compound with the longest carbon-carbon bond is ________.
CH,the compound with the strongest carbon-carbon bond is ________,and the compound with the longest carbon-carbon bond is ________.
(Essay)
4.8/5  (27)
(27)
Based on formal charges,the P-O bond order in POCl3 is expected to be ________.
(Short Answer)
4.9/5  (38)
(38)
In the most acceptable electron-dot structure for carbonyl fluoride,COF2 the central atom is
(Multiple Choice)
4.8/5  (37)
(37)
Compound A is a solid with a melting point of 85°C,and compound B is a gas at 75°C and one atmosphere pressure.Based on these data,one would expect
(Multiple Choice)
4.8/5  (40)
(40)
Arrange the following in order of increasing ionic character: Al2S3,MgS,Na2S,P4S3,S8.
(Multiple Choice)
4.7/5  (37)
(37)
Two resonance forms for SOCl2 are given below. 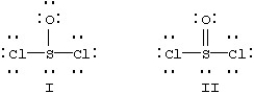 Which is favored by the octet rule and which by formal charge considerations?
Which is favored by the octet rule and which by formal charge considerations?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following are allowed resonance forms of NCS-? I [: N ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c5_7145_a2f7_2d1c6fb99396_TB4940_11.jpg) C -
C - ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c5_7146_a2f7_373eb22ef1c5_TB4940_11.jpg) :] - and [:
:] - and [: ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c5_9857_a2f7_079af94b3bc3_TB4940_11.jpg) = C =
= C = ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c5_9858_a2f7_712ed6cb2e0d_TB4940_11.jpg) :] -
II [: N
:] -
II [: N ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c5_bf69_a2f7_1503286218fd_TB4940_11.jpg) C -
C - ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c5_bf6a_a2f7_3b7cb00b2527_TB4940_11.jpg) :] - and [: N
:] - and [: N ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c5_bf6b_a2f7_63adc7e3eb54_TB4940_11.jpg) C =
C = ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c5_e67c_a2f7_51f22e6ff12d_TB4940_11.jpg) :] -
III [: N
:] -
III [: N ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c5_e67d_a2f7_63c442c8d6dc_TB4940_11.jpg) C -
C - ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c6_0d8e_a2f7_9f8c7794f49a_TB4940_11.jpg) :] - and [:
:] - and [: ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c6_0d8f_a2f7_dfed915d583d_TB4940_11.jpg) - C
- C ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup>](https://storage.examlex.com/TB4940/11ea7e2d_d0c6_34a0_a2f7_49ca047e2680_TB4940_11.jpg) N :] -
N :] -
(Multiple Choice)
4.9/5  (38)
(38)
The electronegativities for the elements vary from 0.7 for cesium to 4.0 for fluorine.The electronegativity for iodine is 2.5.Based entirely on the general guidelines for electronegativities and bond character,
(Multiple Choice)
4.8/5  (35)
(35)
Based on formal charge considerations,the electron-dot structure of CO32- ion has
(Multiple Choice)
4.8/5  (44)
(44)
When melting S8,________ forces must be overcome and S8 is expected to have a ________ melting point than MgS.
(Multiple Choice)
4.9/5  (32)
(32)
The Lewis electron-dot structure of H2CO has ________ nonbonding electron pairs,________ bonding electron pairs,and a carbon-oxygen bond order of ________.
(Short Answer)
4.8/5  (41)
(41)
Which of the following molecules is expected to have the highest melting point?
(Multiple Choice)
4.8/5  (28)
(28)
Assign formal charges to each atom in the resonance form for SOCl2 given below. 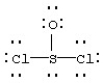
(Multiple Choice)
4.8/5  (40)
(40)
Of the bonds C-C,C-N,C-O,and C-F,the bond that is most polar is ________.
(Short Answer)
4.8/5  (41)
(41)
How many lone pairs in the correct electron dot structure of O3?
(Multiple Choice)
4.9/5  (30)
(30)
How many lone pairs of electrons are on the N atom in NBr3?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 41 - 60 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)