Exam 5: Periodicity and the Electronic Structure of Atoms
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
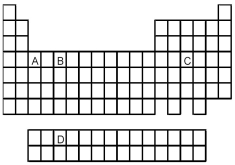
Atoms of which element,indicated by letter on the periodic table,have the orbital-filling diagram shown below? 
Free
(Multiple Choice)
4.8/5  (47)
(47)
Correct Answer:
D
An oxygen molecule has a mass of 5.3 × 10-26 kg and an approximate diameter of 3.6 × 10-10 m.If the molecule is moving at 400 m/s (1000 mph)with an uncertainty in velocity of 1 m/s,the uncertainty in position
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
D
The number of orbitals in the n = 3 shell is ________.
Free
(Short Answer)
4.8/5  (43)
(43)
Correct Answer:
9
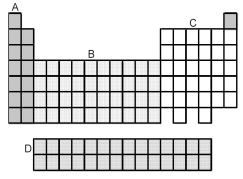 -Which grouping of elements,indicated by letter on the periodic table above,represents the f-block elements?
-Which grouping of elements,indicated by letter on the periodic table above,represents the f-block elements?
(Multiple Choice)
4.7/5  (38)
(38)
The first vibrational level for NaH lies at 1.154 × 10-20 J and the second vibrational level lies at 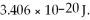 What is the frequency of the photon emitted when a molecule of NaH drops from the second vibrational level to the first vibrational level?
What is the frequency of the photon emitted when a molecule of NaH drops from the second vibrational level to the first vibrational level?
(Multiple Choice)
4.8/5  (23)
(23)
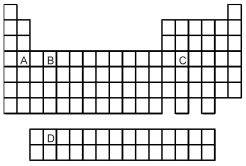 -Which element,indicated by letter on the periodic table above,contains one f electrons?
-Which element,indicated by letter on the periodic table above,contains one f electrons?
(Multiple Choice)
4.9/5  (33)
(33)
What are the possible values of n and ml for an electron in a 6d orbital?
(Multiple Choice)
4.9/5  (34)
(34)
Which group of elements,indicated by letter on the periodic table,has electrons with the ground-state valence-shell electron configuration ns2 np4? 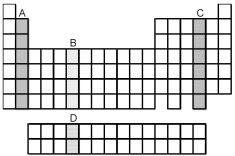
(Multiple Choice)
4.8/5  (34)
(34)
The wave characteristics of a large,moving object,such as an automobile,are difficult to observe because the
(Multiple Choice)
4.8/5  (42)
(42)
The spheres below represent atoms of Li,Be,B,and F (not necessarily in that order). 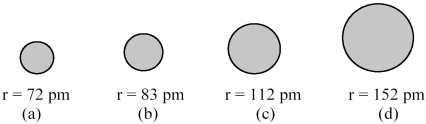 -Which one of these spheres represents an atom of Be?
-Which one of these spheres represents an atom of Be?
(Multiple Choice)
4.7/5  (38)
(38)
An old copper penny has a mass 3 × 1022 times that of a copper atom.Compare the de Broglie wavelength of a penny moving at 0.5 m/s to that of a copper atom moving 104 times as fast.The wavelength for the
(Multiple Choice)
4.9/5  (34)
(34)
The property of a wave that is associated with brightness or intensity is ________.
(Short Answer)
4.7/5  (39)
(39)
For a multielectron atom,a 3s orbital lies lower in energy than a 3p orbital because
(Multiple Choice)
4.9/5  (42)
(42)
Which groups of elements,indicated by letter on the periodic table,have two unpaired p electrons in their valence shell? 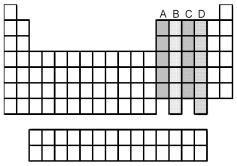
(Multiple Choice)
4.8/5  (39)
(39)
 -Which grouping of elements,indicated by letter on the periodic table above,represents the d-block elements?
-Which grouping of elements,indicated by letter on the periodic table above,represents the d-block elements?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following represent electron configurations that violate the Pauli exclusion principle? (A)[Ne]3s13p5 (B)[Kr]4d125s25p6 (C)[Ar]3d104s24p2
(Multiple Choice)
4.9/5  (37)
(37)
The subshell designations follow the alphabet after f.What is the first shell in which an h orbital would be allowed?
(Multiple Choice)
4.7/5  (43)
(43)
Showing 1 - 20 of 168
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)