Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
How many chloride ions are in 1.50 mol of aluminum chloride?
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
D
Which one of the following contains 38.7% carbon by mass?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
C
How many moles of O2 react with 3.600 mol of Cu2O in the following reaction? 2 Cu2O(s)+ O2(g)→ 4 CuO(s)
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
C
If the percent yield for the following reaction is 80.0%,how many grams of KClO3 are needed to produce 42.0 g of O2? 2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
(Multiple Choice)
4.8/5  (35)
(35)
When the reaction C3H8 + O2 → CO2 + H2O is balanced,the total number of oxygen atoms in the balanced equation is ________.
(Short Answer)
4.8/5  (30)
(30)
What is the empirical formula of a substance that contains 12.0 g of C,2.00 g of H,and 5.33 g of O?
(Multiple Choice)
5.0/5  (39)
(39)
In the combustion analysis of an unknown compound containing only carbon,hydrogen,and oxygen,the grams of oxygen are found from the grams of
(Multiple Choice)
4.8/5  (29)
(29)
Consider the following balanced reaction. 4A + 2B → 2C + D
8.0 moles of A and 30.moles of B react to form 2.5 moles of C.What is the percent yield of this reaction?
(Multiple Choice)
4.8/5  (37)
(37)
A compound responsible for the odor of garlic has a molecular weight of 146 g/mol.A 0.650 g sample of the compound contains 0.321 g of carbon,0.044 g of hydrogen,and 0.285 g of sulfur.What is the molecular formula of the compound?
(Multiple Choice)
4.8/5  (37)
(37)
When 12.00 g of calcium metal is reacted with water,12.00 g of calcium hydroxide is produced.Using the following balanced equation,calculate the percent yield for the reaction? Ca(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ H2(g)
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following statements about mass spectrometry is false?
(Multiple Choice)
4.9/5  (34)
(34)
9.0 g of iron is reacted with 9.0 g of water according to the chemical equation shown below.Which one of the following statements is false? 3 Fe(s)+ 4 H2O(l)→ Fe3O4(s)+ 4 H2(g)
(Multiple Choice)
4.9/5  (33)
(33)
Reaction of A (unshaded spheres)with B2 (shaded spheres)is shown schematically in the following diagram.Which equation best describes the stoichiometry of the reaction? 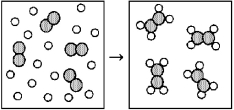
(Multiple Choice)
4.8/5  (26)
(26)
10 g of nitrogen is reacted with 5.0 g of hydrogen to produce ammonia according to the chemical equation shown below.Which one of the following statements is false?
N2(g)+ 3 H2(g)→ 2 NH3(g)
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 159
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)