Exam 20: Transition Elements and Coordination Chemistry
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
What is the crystal field energy level diagram for the complex [Co(CN)6]3-? ![What is the crystal field energy level diagram for the complex [Co(CN)<sub>6</sub>]<sup>3-</sup>?](https://storage.examlex.com/TB4940/11ea7e2d_d1c9_73a6_a2f7_d5381e631ad4_TB4940_00.jpg)
(Multiple Choice)
4.9/5  (31)
(31)
Which ligand when bonded to a metal would be incorrectly named?
(Multiple Choice)
5.0/5  (34)
(34)
The compounds [Cr(H2O)6]Cl3 and [CrCl3(H2O)3] ∙ 3H2O are examples of
(Multiple Choice)
4.9/5  (37)
(37)
What statement is inconsistent with the chemistry of iron?
(Multiple Choice)
4.9/5  (41)
(41)
What statement is most inconsistent with the chemistry of transition elements?
(Multiple Choice)
4.8/5  (38)
(38)
Based on the variation in Zeff,which oxoanion should be the weakest oxidizing agent?
(Multiple Choice)
4.8/5  (41)
(41)
The short hand electron configuration of iron in [FeF6]3- is ________.
(Short Answer)
4.8/5  (34)
(34)
Consider the following ethylenediamine complexes.The black sphere represents Co,gray connected spheres represent ethylenediamine,NH2CH2CH2NH2,and unshaded spheres represent Br. 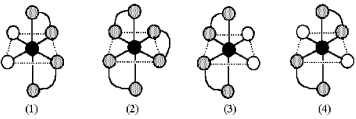 -Which complexes are chiral?
-Which complexes are chiral?
(Multiple Choice)
4.7/5  (41)
(41)
A complex ion that has a broad absorption band at 530 nm in its visible absorption spectrum will appear to be
(Multiple Choice)
4.9/5  (35)
(35)
What is the compound responsible for the green patina seen on bronze monuments?
(Multiple Choice)
4.9/5  (34)
(34)
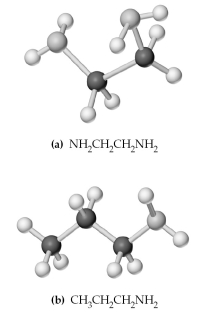
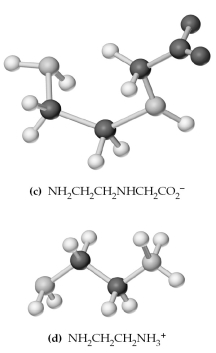 -Which of the above are bidentate or tridentate ligands,capable of forming chelate rings?
-Which of the above are bidentate or tridentate ligands,capable of forming chelate rings?
(Multiple Choice)
4.9/5  (28)
(28)
The element that forms a 2+ ion with the electron configuration [Xe] 4f14 5d8 is ________.
(Short Answer)
4.8/5  (38)
(38)
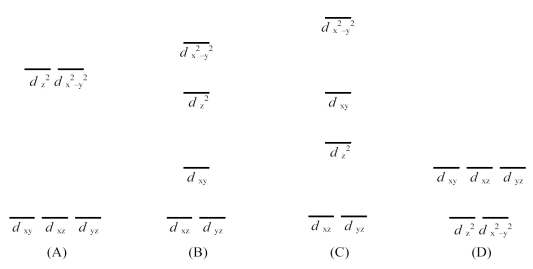 -Which is the crystal field energy level diagram for an octahedral ML6 complex?
-Which is the crystal field energy level diagram for an octahedral ML6 complex?
(Multiple Choice)
4.8/5  (29)
(29)
For an octahedral complex what metal d orbitals are directly towards the ligands?
(Multiple Choice)
4.7/5  (39)
(39)
Transition elements are located in the ________-block of the periodic table.
(Short Answer)
4.9/5  (43)
(43)
The third transition series metals are shown below. 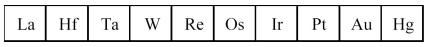 -Which has the greatest density?
-Which has the greatest density?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 101 - 120 of 190
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)