Exam 20: Transition Elements and Coordination Chemistry
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
The complex cis-[CoCl(NH3)(NH2CH2CH2NH2)2]2+ was resolved into optical isomers in 1911 by Alfred Werner,demonstrating the octahedral geometry of the ion.Name this complex ion.
(Multiple Choice)
4.9/5  (36)
(36)
What is the ground-state electron configuration for the element nickel (Z = 28)?
(Multiple Choice)
4.9/5  (41)
(41)
How many unpaired electrons are present in the high spin form of the [CoF6]3- complex and what metal orbitals are used in bonding?
(Multiple Choice)
4.8/5  (44)
(44)
In order to form a neutral compound,hexafluoroaluminate would require
(Multiple Choice)
4.8/5  (42)
(42)
What statement is inconsistent with the chemistry of copper?
(Multiple Choice)
4.7/5  (47)
(47)
What statement is inconsistent with the crystal field theory of tetrahedral complexes?
(Multiple Choice)
4.8/5  (42)
(42)
For transition elements,which of the following occurs as the effective nuclear charge increases?
(Multiple Choice)
4.8/5  (36)
(36)
The second transition series metals are shown below. 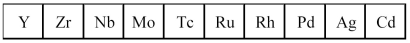 -Which has the largest atomic radius?
-Which has the largest atomic radius?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following chromium species is the strongest acid?
(Multiple Choice)
4.8/5  (40)
(40)
Write the chemical formula for aquabromobis(ethylenediamine)chromium(III)chloride.
(Multiple Choice)
4.8/5  (36)
(36)
The color exhibited by coordination compounds is usually due to the absorption of light by a d-electron,resulting in the promotion of the d-electron from its ground-state d-orbital to a higher energy orbital.The first transition series element expected to have a colorless aqueous solution M2+ ion is ________.
(Short Answer)
4.9/5  (25)
(25)
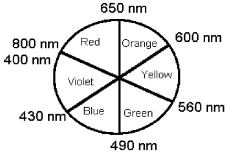 -Using the above schematic of an artist's color wheel,determine the color seen if a substance absorbs red and yellow light.
-Using the above schematic of an artist's color wheel,determine the color seen if a substance absorbs red and yellow light.
(Multiple Choice)
4.7/5  (41)
(41)
What is the characteristic outer electron configuration for transition elements?
(Multiple Choice)
4.9/5  (46)
(46)
Showing 21 - 40 of 190
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)