Exam 14: The Ideal Gas Law and Kinetic Theory
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
An ideal gas with a fixed number of molecules is maintained at a constant pressure. At 30.0 °C, the volume of the gas is 1.25 m3. What is the volume of the gas when the temperature is increased to 150.0 °C?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
C
Note the following six properties: 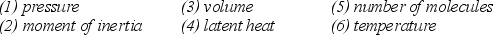 Which four of the listed properties are needed to describe an ideal gas?
Which four of the listed properties are needed to describe an ideal gas?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
B
In the space between two stars, the temperature of a gas cloud is 12 K; and the density of the gas is 1.2 × 10-8 atom/m3. What is the absolute pressure of the gas?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
An automobile tire is inflated to a gauge pressure of 32 lb/in2 at a temperature of -10.0 °C. Under strenuous driving, the tire heats up to 40.0 °C. What is the new gauge pressure if the volume of the tire remains essentially the same? (atmospheric pressure = 14.7 lb/in2)
(Multiple Choice)
4.8/5  (34)
(34)
An ideal gas is confined within a closed cylinder at a pressure of 2.026 × 105 Pa by a piston. The piston moves until the volume of the gas is reduced to one-ninth of the initial volume. What is the final pressure of the gas when its temperature returns to its initial value?
(Multiple Choice)
4.7/5  (38)
(38)
How many air molecules are in a room at temperature 23.8 °C and standard pressure if the dimensions of the room are 3.44 m × 3.26 m × 2.28 m?
(Multiple Choice)
4.9/5  (36)
(36)
A silver coin has a mass of 17.75 g. If the atomic mass of silver is 107.868 u, how many silver atoms are in the coin?
(Multiple Choice)
4.9/5  (37)
(37)
Which one of the following statements concerning a collection of gas molecules at a certain temperature is true?
(Multiple Choice)
4.8/5  (29)
(29)
What is the density of methane, CH4, (molecular mass = 16 u) at STP?
(Multiple Choice)
4.8/5  (33)
(33)
Complete the following statement: The absolute temperature of an ideal gas is directly proportional to
(Multiple Choice)
4.9/5  (35)
(35)
A flask contains 1.00 mole of oxygen gas, O2, at 0.00 °C and 1.013 × 105 Pa. What is the rms speed of the molecules? Note: the atomic mass of O is 16 u.
(Multiple Choice)
4.8/5  (46)
(46)
How many molecules are in 0.044 kg of carbon dioxide, CO2? (atomic masses: C = 12 u; O = 16 u)
(Multiple Choice)
4.8/5  (29)
(29)
A tank contains 135 moles of the monatomic gas argon at a temperature of 15.3 °C. How much energy must be added to the gas to increase its temperature to 45.0 °C?
(Multiple Choice)
4.8/5  (27)
(27)
Which one of the following statements concerning the mole is false?
(Multiple Choice)
4.8/5  (36)
(36)
On a cold day (-3 °C), the gauge pressure on a tire is 2.0 atm. If the tire is heated to 27 °C, without changing its volume, what will the gauge pressure be?
(Multiple Choice)
4.8/5  (32)
(32)
Complete the following statement: The atomic mass unit (u) is defined so that 1 u is exactly equal to the mass of
(Multiple Choice)
4.8/5  (31)
(31)
Heat is supplied to a sample of a monatomic ideal gas at 40 °C. It is observed that the gas expands until its volume and pressure are doubled. What is the final temperature of the gas?
(Multiple Choice)
4.8/5  (37)
(37)
Neon gas at 305 K is confined within a constant volume at a pressure P1. If the gas has a pressure P2 when it is cooled to 125 K, what is the ratio of P2 to P1?
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the following statements concerning diffusion is true?
(Multiple Choice)
4.9/5  (36)
(36)
Which one of the following factors is directly responsible for the pressure exerted by a confined gas?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 1 - 20 of 55
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)