Exam 30: The Nature of the Atom
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
The ground state electronic configuration of a neon atom is 1s2 2s2 2p6. How many of these electrons have magnetic quantum number ml = 0?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
C
Determine the maximum wavelength of incident radiation that can be used to remove the remaining electron from a singly ionized helium atom He+ (Z = 2). Assume the electron is in its ground state.
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
C
Complete the following statement: An h subshell refers to orbital quantum number
Free
(Multiple Choice)
5.0/5  (38)
(38)
Correct Answer:
E
An electron in an atom has the following set of quantum numbers:
n = 2, 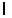 = 1,
= 1, 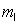 = -1, ms = +1/2.
-In which subshell can the electron be found?
= -1, ms = +1/2.
-In which subshell can the electron be found?
(Multiple Choice)
4.9/5  (35)
(35)
Use the Bohr model to estimate the K X-ray wavelength for a gold atom (Z = 79).
(Multiple Choice)
4.8/5  (39)
(39)
The figure shows an energy level diagram for the hydrogen atom. Several transitions are shown and are labeled by letters. 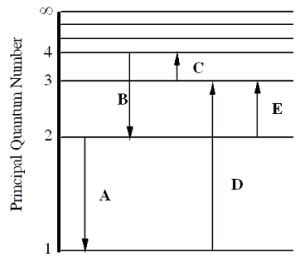 Note: The diagram is not drawn to scale.
-Determine the wavelength of the radiation involved in transition B.
Note: The diagram is not drawn to scale.
-Determine the wavelength of the radiation involved in transition B.
(Multiple Choice)
4.7/5  (35)
(35)
Complete the following statement: An individual copper atom emits electromagnetic radiation with wavelengths that are
(Multiple Choice)
4.9/5  (35)
(35)
Complete the following sentence: In the condition known as population inversion,
(Multiple Choice)
4.7/5  (26)
(26)
How many electron states (including spin states) are possible in a hydrogen atom if its energy is -3.4 eV?
(Multiple Choice)
4.8/5  (28)
(28)
Determine the kinetic energy of an electron that has a de Broglie wavelength equal to twice the diameter of the hydrogen atom. Assume that the hydrogen atom is a sphere of radius 5.3 × 10-11 m.
(Multiple Choice)
4.9/5  (28)
(28)
What is the longest wavelength in the Paschen series of atomic spectra?
(Multiple Choice)
4.8/5  (38)
(38)
In the planetary model of the atom where electrons orbit a centralized nucleus, what is the approximate ratio of the radius of the nucleus to that of the electron orbits, rn/re?
(Multiple Choice)
4.9/5  (39)
(39)
In an X-ray tube, electrons with energy 38 keV are incident on a cobalt (Z = 27) target. Determine the cutoff wavelength for X-ray production.
(Multiple Choice)
4.9/5  (39)
(39)
The figure shows an energy level diagram for the hydrogen atom. Several transitions are shown and are labeled by letters.  Note: The diagram is not drawn to scale.
-In which transition is a Balmer series photon absorbed?
Note: The diagram is not drawn to scale.
-In which transition is a Balmer series photon absorbed?
(Multiple Choice)
4.8/5  (23)
(23)
A pulsed laser has an average output power of 4.0 W. Each pulse consists of light at wavelength 5.0 × 10-7 m and has a 25 ms duration. How many photons are emitted in a single pulse?
(Multiple Choice)
4.8/5  (28)
(28)
What is the shortest possible wavelength in the Lyman series for atomic hydrogen?
(Multiple Choice)
4.8/5  (35)
(35)
Which model of atomic structure was developed to explain the results of the experiment shown? 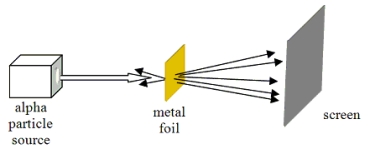
(Multiple Choice)
4.7/5  (31)
(31)
Which one of the following electronic configurations corresponds to an atomic ground state?
(Multiple Choice)
4.8/5  (36)
(36)
Why was it necessary for Bohr to require that electrons remain in stationary orbits?
(Multiple Choice)
4.8/5  (37)
(37)
An atom will emit photons when one of its electrons goes from
(Multiple Choice)
4.9/5  (30)
(30)
Showing 1 - 20 of 74
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)