Exam 15: Thermodynamics
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
A gasoline engine with an efficiency of 0.40 generates 1800 W of power. If a liter of gasoline has an energy content of 3.7 × 107 J, how many liters of gasoline does the engine consume each hour?
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
B
What is the maximum possible efficiency of an engine operating between the boiling and freezing points of water at sea level?
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
A
A match ignites within in an oxygen-filled cylinder that has a movable piston. The piston is moved so quickly that no heat escapes. What kind of change is demonstrated in this process?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
B
Heat is added to a sample of an ideal monatomic gas. Which one of the following statements is necessarily true?
(Multiple Choice)
4.8/5  (31)
(31)
An ideal monatomic gas expands isothermally from state A to state B. The gas then cools at constant volume to state C. The gas is then compressed isobarically to D before it is heated until it returns to state A. 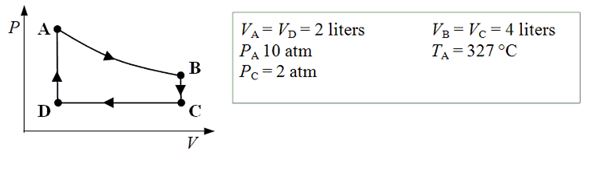 -How much work is done on the gas as it is compressed isobarically from state C to state D?
-How much work is done on the gas as it is compressed isobarically from state C to state D?
(Multiple Choice)
4.9/5  (25)
(25)
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C. The water freezes and comes into thermal equilibrium with the interior of the freezer.
-Which one of the following statements is true concerning this process?
(Multiple Choice)
4.8/5  (31)
(31)
Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi. The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi.
-What is the change in entropy of the sample?
(Multiple Choice)
4.8/5  (39)
(39)
What change in temperature occurs when 1600 J of heat are removed from 3.0 moles of monatomic gas under constant pressure?
(Multiple Choice)
4.9/5  (31)
(31)
5.00 kg of liquid water is heated to 100.0 °C in a closed system. At this temperature, the density of liquid water is 958 kg/m3. The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa. A moveable piston of negligible weight rests on the surface of the water. The water is then converted to steam by adding an additional amount of heat to the system. When all of the water is converted, the final volume of the steam is 8.50 m3. The latent heat of vaporization of water is 2.26 × 106 J/kg.  -How much heat is added to the system in the isothermal process of converting all of the water into steam?
-How much heat is added to the system in the isothermal process of converting all of the water into steam?
(Multiple Choice)
4.8/5  (38)
(38)
Which one of the following situations is a direct application of the Zeroth Law of Thermodynamics?
(Multiple Choice)
4.8/5  (34)
(34)
An ideal monatomic gas expands isobarically from state A to state B. It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.
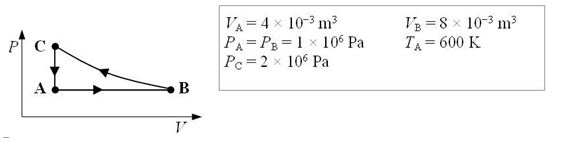 -What is the temperature of the gas when it is in state B?
-What is the temperature of the gas when it is in state B?
(Multiple Choice)
4.7/5  (35)
(35)
In a reversible heat engine, one mole of an ideal gas is carried through a circular cycle beginning and ending at point A as shown in the figure. Which one of the following statements concerning this system is false? 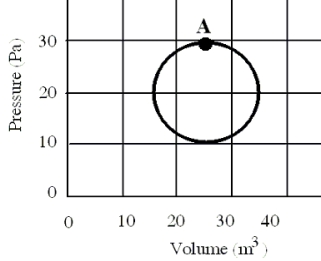
(Multiple Choice)
5.0/5  (39)
(39)
Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi. The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi.
-Which one of the following expressions represents the final pressure of the gas?
(Multiple Choice)
4.8/5  (30)
(30)
A thermally isolated sample of an ideal gas at a fixed temperature is confined to one half of a container by an impermeable membrane. The other half of the container is evacuated. The membrane is then pierced and the gas is allowed to expand freely and to double its volume as shown. Which one of the following statements is true concerning this situation? 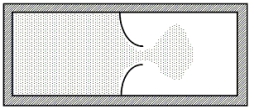
(Multiple Choice)
4.8/5  (27)
(27)
A Carnot engine operates between hot and cold reservoirs with temperatures 527 °C and -73.0 °C, respectively. If the engine performs 1000.0 J of work per cycle, how much heat is extracted per cycle from the hot reservoir?
(Multiple Choice)
4.9/5  (33)
(33)
Enclosed beneath the moveable piston in the drawing is 4.8 moles of a monatomic ideal gas. The gas performs work on the piston as 2300 J of heat are added from the surroundings. During the process, the temperature of the gas decreases by 45 K. How much work does the gas perform? 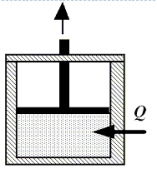
(Multiple Choice)
4.8/5  (38)
(38)
A quantity of carbon monoxide gas is slowly compressed adiabatically in an insulated container to one-half of its initial volume. The ratio of the specific heat capacities at constant pressure and constant volume, cP/cV , for carbon dioxide is approximately 1.3. Determine the final pressure of the gas if the initial pressure is 2.0 × 105 Pa.
(Multiple Choice)
4.7/5  (39)
(39)
An ideal monatomic gas expands isobarically from state A to state B. It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.
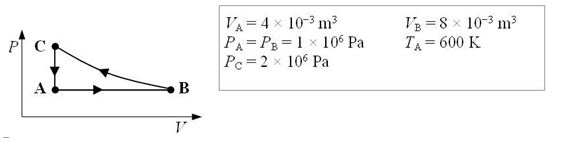 -How much work is done by the gas in expanding isobarically from A to B?
-How much work is done by the gas in expanding isobarically from A to B?
(Multiple Choice)
4.9/5  (45)
(45)
Which one of the following statements best describes the operation of a heat engine?
(Multiple Choice)
4.8/5  (41)
(41)
A container is divided into two chambers that are separated by a valve. The left chamber contains one mole of a monatomic ideal gas. The right chamber is evacuated. At some instant, the valve is opened and the gas rushes freely into the right chamber. Which one of the following statements concerning this process is true? 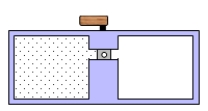
(Multiple Choice)
4.9/5  (32)
(32)
Showing 1 - 20 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)