Exam 20: The First Law of Thermodynamics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion46 Questions
Exam 17: Sound Waves48 Questions
Exam 18: Superposition and Standing Waves60 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields83 Questions
Exam 24: Gausss Law80 Questions
Exam 25: Electric Potential97 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field105 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 36: Image Formation46 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure50 Questions
Exam 45: Applications of Nuclear Physics40 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
If a person in Alaska were locked out of his house on a day when the temperature outside was −40°C,his thick clothing would mostly reduce the loss of thermal energy by
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
A
A 100 kg marble slab falls off a skyscraper and falls 200 m to the ground without hitting anyone.Its fall stops within milliseconds,so that there is no loss of thermal energy to its surroundings if its temperature is measured immediately after it stops.By how much has its temperature changed as a result of the fall,if we ignore energy gained or lost as a result of its interaction with the atmosphere? ( 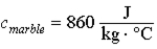 ) )
) )
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
C
100 grams of liquid nitrogen at 77 K is stirred into a beaker containing 200 grams of 5°C water.If the nitrogen leaves the solution as soon as it turns to gas,how much water freezes? (The heat of evaporation of nitrogen is 6.09 cal/gram and the heat of fusion of water is 80 cal/gram. )
Free
(Short Answer)
4.9/5  (35)
(35)
Correct Answer:
none
We are able to define a mechanical equivalent for heat because
(Multiple Choice)
4.9/5  (39)
(39)
Aluminum rod #1 is 1.0 m long with cross-section area 2.0 cm2.Aluminum rod #2 is 2.0 m long with cross-section area 4.0 cm2.Aluminum rod #3 is 3.0 m long with cross-section area 9.0 cm2.If each rod has the same temperature difference applied across its ends,which rod has the greatest rate of transfer of energy between its ends?
(Multiple Choice)
4.8/5  (35)
(35)
An 8 000-kg aluminum flagpole 100-m long is heated by the sun from a temperature of 10°C to 20°C.Find the heat transferred (in J)to the aluminum if the specific heat of aluminum is 0.215 cal/g⋅°C.
(Multiple Choice)
4.7/5  (35)
(35)
A super-insulated house is at a temperature of 20°C.The temperature outside is 0°C.The surface area of the house is 200 m2,and the emissivity is 1.Approximately how much energy is radiated (in W)per second?
(Multiple Choice)
4.9/5  (35)
(35)
Which statement below regarding the First Law of Thermodynamics is most correct?
(Multiple Choice)
4.9/5  (35)
(35)
Star A has a radius of 200 000 km and a surface temperature of 6 000 K.Star B has a radius of 400 000 km and a surface temperature of 3 000 K.The emissivity of both stars is the same.What is the ratio of the rate of energy radiated by Star A to that of Star B?
(Short Answer)
4.7/5  (38)
(38)
A team of people who traveled to the North Pole by dogsled lived on butter because they needed to consume 6 000 dietician's Calories each day.Because the ice there is lumpy and irregular,they had to help the dogs by pushing and lifting the load.Assume they had a 16 hour working day and that each person could lift a 500 N load.How many times would a person have to lift this weight 1.00 m upwards in a constant gravitational field where 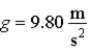 To do the work equivalent to 6 000 Calories?
To do the work equivalent to 6 000 Calories?
(Multiple Choice)
4.8/5  (36)
(36)
The work done in the expansion from an initial to a final state
(Multiple Choice)
4.8/5  (29)
(29)
Duff states that equal masses of all substances have equal changes in internal energy when they have equal changes in temperature.Javan states that the change in internal energy is equal to a constant times the change in temperature for every ΔT,no matter how large or how small ΔT is,but that the constant is different for different substances.Which one,if either,is correct?
(Multiple Choice)
4.8/5  (32)
(32)
An 8 000-kg aluminum flagpole 100-m high is heated by the sun from a temperature of 10°C to 20°C.Find the increase in internal energy (in J)of the aluminum.(The coefficient of linear expansion is 24 × 10−6 (°C)−1,the density is 2.7 × 103 kg/m3,and the specific heat of aluminum is 0.215 cal/g⋅°C. )
(Multiple Choice)
4.9/5  (35)
(35)
A 100-kg student eats a 200-Calorie doughnut.To "burn it off",he decides to climb the steps of a tall building.How high (in m)would he have to climb to expend an equivalent amount of work? (1 food Calorie = 103 calories. )
(Multiple Choice)
4.7/5  (33)
(33)
Two kilograms of water at 100°C is converted to steam at 1 atm.Find the work done (in J).(The density of steam at 100°C is 0.598 kg/m3. )
(Multiple Choice)
4.9/5  (32)
(32)
A 300-g glass thermometer initially at 25°C is put into 200 cm3 of hot water at 95°C.Find the final temperature (in °C)of the thermometer,assuming no heat flows to the surroundings.(The specific heat of glass is 0.2 cal/g⋅°C. )
(Multiple Choice)
4.8/5  (40)
(40)
A slab of concrete and an insulating board are in thermal contact with each other.The temperatures of their outer surfaces are 68°F and 50°F.Determine the rate of heat transfer (in BTU/ft2⋅h)if the R values are 1.93 and 8.7 ft2⋅°F⋅h/BTU,respectively.
(Multiple Choice)
4.9/5  (32)
(32)
One gram of water is heated from 0°C to 100°C at a constant pressure of 1 atm.Determine the approximate change in internal energy (in cal)of the water.
(Multiple Choice)
4.9/5  (34)
(34)
If an object feels cold to the touch,the only statement that you can make that must be correct is that
(Multiple Choice)
4.9/5  (36)
(36)
Showing 1 - 20 of 61
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)