Exam 21: The Kinetic Theory of Gases
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion46 Questions
Exam 17: Sound Waves48 Questions
Exam 18: Superposition and Standing Waves60 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields83 Questions
Exam 24: Gausss Law80 Questions
Exam 25: Electric Potential97 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field105 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 36: Image Formation46 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure50 Questions
Exam 45: Applications of Nuclear Physics40 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
The air in an automobile engine at 20°C is compressed from an initial pressure of 1.0 atm and a volume of 200 cm3 to a volume of 20 cm3.Find the temperature if the air behaves like an ideal gas (γ = 1.4)and the compression is adiabatic.
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
B
During an adiabatic compression,a volume of air decreases to 1/4 its original size.Calculate its final pressure if its original pressure was 1 atm.(Assume the air behaves like an ideal gas with γ = 1.4. )
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
A
Two tanks of gas,one of hydrogen,H2,and one of helium,He,contain equal numbers of moles of gas.The gram-molecular mass of He is twice that of H2.Both tanks of gas are at the same temperature,293 K.Which statement(s)below is(are)correct when we ignore vibrational motion?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
D
The root mean square speed of a gas molecule is greater than the average speed,because the former gives a greater weight to
(Multiple Choice)
4.9/5  (29)
(29)
The molar specific heat at constant volume at 0°C of an ideal diatomic gas is
(Multiple Choice)
4.8/5  (44)
(44)
One mole of helium gas expands adiabatically from 2 atm pressure to 1 atm pressure.If the original temperature of the gas is 20°C,what is the final temperature of the gas? (γ = 1.67)
(Short Answer)
4.9/5  (26)
(26)
Nitrogen gas is heated by a pulsed laser to 50 000 K.If the diameter of the nitrogen atoms is assumed to be 1.0 × 10−10 m,and the pressure is 1.0 atm,what is the mean free path?
(Multiple Choice)
4.8/5  (36)
(36)
If the rms speed of helium atoms is vrms,He at temperature T,what is the rms speed of CO2 at the same temperature?
(Multiple Choice)
4.8/5  (32)
(32)
The average kinetic energy of a nitrogen molecule at room temperature (20°C)is
(Multiple Choice)
4.9/5  (37)
(37)
The theorem of equipartition of energy states that the energy each degree of freedom contributes to each molecule in the system (an ideal gas)is
(Multiple Choice)
4.9/5  (30)
(30)
The average molecular translational kinetic energy of a molecule in an ideal gas is
(Multiple Choice)
4.8/5  (33)
(33)
The relation PV = nRT holds for all ideal gases.The additional relation PVγ holds for an adiabatic process.The figure below shows two curves: one is an adiabat and one is an isotherm.Each starts at the same pressure and volume.Which statement is correct? (Note: "∝" means "is proportional to". ) 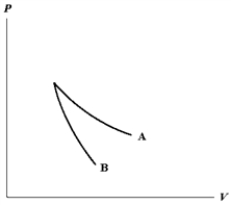
(Multiple Choice)
4.7/5  (35)
(35)
Assume molecules have an average diameter of 3.00 × 10−10 m.How many times larger is the mean free path than the diameter at one atmosphere and 0°C?
(Multiple Choice)
4.8/5  (38)
(38)
The molar specific heat at constant volume at 0°C of an ideal monatomic gas is
(Multiple Choice)
4.9/5  (35)
(35)
The molar specific heat at constant pressure at 0°C of an ideal monatomic gas is
(Multiple Choice)
4.9/5  (31)
(31)
An ideal gas is allowed to expand adiabatically until its volume increases by 50%.By approximately what factor is the pressure reduced? (γ = 5/3. )
(Multiple Choice)
4.8/5  (36)
(36)
During the volcanic eruption of Mt.Pelee in 1902,an incredibly hot "burning cloud" rolled down the mountain and incinerated the town of Saint-Pierre.From the damage done,the temperature in the cloud was estimated at 700°C.If the air temperature was 20°C and a mole of air is 29 grams,estimate the molecular weight of the gas in the "burning cloud" that made it heavier than the surrounding air.(As a follow-on,estimate the most probable composition of the cloud.Some typical volcanic gases are H2S,SO2,H2SO4,CO2,NO. )
(Short Answer)
4.8/5  (38)
(38)
If CP for an ideal gas is 35.4 J/mol⋅K,which of the following is CV for this gas?
(Multiple Choice)
4.7/5  (29)
(29)
A 50-gram sample of dry ice (solid CO2)is placed in a 4-liter container.The system is sealed and allowed to reach room temperature (20°C).By approximately how much does the pressure inside the container increase when the dry ice turns to gas? (Ignore the initial volume of the sample. )
(Short Answer)
4.9/5  (34)
(34)
Showing 1 - 20 of 38
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)