Exam 42: Atomic Physics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion46 Questions
Exam 17: Sound Waves48 Questions
Exam 18: Superposition and Standing Waves60 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields83 Questions
Exam 24: Gausss Law80 Questions
Exam 25: Electric Potential97 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field105 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 36: Image Formation46 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure50 Questions
Exam 45: Applications of Nuclear Physics40 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
How fast is the electron moving in the first Bohr orbit?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
B
All quantum states forming a sub-shell have the same
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
E
The ground state configuration of chlorine (Z = 17)is
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
B
Suppose a beam of electrons is incident on a collection of hydrogen atoms,all of which are in the lowest energy state (n = 1).What is the minimum energy the electrons can have if they are to excite the hydrogen atoms into the n = 2 state?
(Short Answer)
4.8/5  (41)
(41)
An electron is moving at a speed of 2.1 × 106 m/s in the first Bohr orbit.Determine its de Broglie wavelength.
(Multiple Choice)
4.9/5  (38)
(38)
Light is emitted by hydrogen atoms in the visible range for a hydrogen atom.Its wavelength is 656 nm.What value of n is associated with the light? (RH = 1.097 × 107 m−1)
(Multiple Choice)
4.7/5  (30)
(30)
The energy difference between the upper and lower levels in a certain laser is 1.9593 eV.What is the wavelength of the light emitted by the laser?
(Short Answer)
4.7/5  (33)
(33)
Zeke says that the magnitude of the orbital angular momentum in the hydrogen atom has the value L = 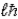 )Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector
)Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector 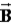 Is
Is 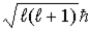 )Which one,if either,is correct,and why?
)Which one,if either,is correct,and why?
(Multiple Choice)
4.8/5  (41)
(41)
Suppose Bohr had chosen the potential energy of the electron in the hydrogen atom to be V = 0 when the electron is in the orbit with n = 1.He could do this by
(Multiple Choice)
4.9/5  (38)
(38)
An electron in a hydrogen atom makes a transition from the n = 3 to the n = 1 energy state.Determine the wavelength of the emitted photon (in nm).
(Multiple Choice)
5.0/5  (27)
(27)
In the subshell of the Li2+ ion with orbital quantum number 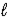 ,the allowed values of the magnetic quantum number
,the allowed values of the magnetic quantum number 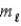 Are
Are
(Multiple Choice)
5.0/5  (36)
(36)
When electrons fill a subshell in which the orbitals have equal energy,the order in which the orbitals are filled is such that
(Multiple Choice)
4.8/5  (37)
(37)
The allowed values of 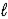 For the n = 3 shell in a Li2+ ion are
For the n = 3 shell in a Li2+ ion are
(Multiple Choice)
4.8/5  (33)
(33)
Of the following states,5s,3p,4f,5p,4g,3d,and 2p,the one which is NOT allowed is
(Multiple Choice)
4.8/5  (36)
(36)
A hydrogen atom is in its first excited state (n = 2).The linear momentum of the electron is (in kg ⋅ m/s)
(Multiple Choice)
4.9/5  (43)
(43)
Adam and Eve are contemplating the beauty of the hydrogen atom.Adam claims that the quantum states with a given value of the principal quantum number n can have any value of the orbital quantum number 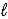 )Eve says that the Snake told her that a state with a given value of
)Eve says that the Snake told her that a state with a given value of 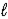 Could have any value of n.Which one,if either,is correct,and why?
Could have any value of n.Which one,if either,is correct,and why?
(Multiple Choice)
4.7/5  (31)
(31)
Showing 1 - 20 of 59
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)