Exam 22: Heat Engines, entropy, and the Second Law of Thermodynamics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion46 Questions
Exam 17: Sound Waves48 Questions
Exam 18: Superposition and Standing Waves60 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields83 Questions
Exam 24: Gausss Law80 Questions
Exam 25: Electric Potential97 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field105 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 36: Image Formation46 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure50 Questions
Exam 45: Applications of Nuclear Physics40 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
When water of mass m and specific heat c is heated from absolute temperature T1 to absolute temperature T2,its change in entropy is
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
A
A vessel containing 5.0 kg of water at 10°C is put into a refrigerator.The 1/7 HP motor (1 HP = 746 W)runs for 5.0 minutes to cool the liquid to the refrigerator's low temperature,0°C.What is the κ of the refrigerator?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
C
Every second at Niagara Falls,some 5000 m3 of water falls a distance of 50 m.What is the increase in entropy per second due to the falling water? (Assume a 20°C environment).
Free
(Short Answer)
4.8/5  (31)
(31)
Correct Answer:
8360 kJ/K
A heat engine absorbs 2 500 J of heat from a hot reservoir and expels 1 000 J to a cold reservoir.When it is run in reverse,with the same reservoirs,the engine pumps 2 500 J of heat to the hot reservoir,requiring 1 500 J of work to do so.Find the ratio of the work done by the heat engine to the work done by the pump.Is the heat engine reversible?
(Multiple Choice)
4.9/5  (41)
(41)
Ten kilograms of water at 0°C is mixed with 10 kg of water at 100°C.The change in entropy (in cal/K)of the system is
(Multiple Choice)
4.9/5  (36)
(36)
Selena states that 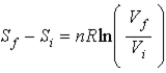 Proves that entropy has a definite value at the beginning and end of an adiabatic free expansion.Ron says
Proves that entropy has a definite value at the beginning and end of an adiabatic free expansion.Ron says 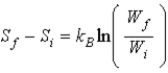 ,where W is the number of microstates of a given macrostate.Which one,if either,is correct?
,where W is the number of microstates of a given macrostate.Which one,if either,is correct?
(Multiple Choice)
4.7/5  (43)
(43)
Determine the change in entropy (in J/K)when 5.00 moles of an ideal gas at 0°C are compressed isothermally from an initial volume of 100 cm3 to a final volume of 20 cm3.
(Multiple Choice)
4.9/5  (27)
(27)
An ideal gas is allowed to expand adiabatically.Assume the process is reversible.The change in entropy is
(Multiple Choice)
4.8/5  (36)
(36)
A lawn mower has a 6-horsepower engine (1 HP = 750 W).If the engine has an efficiency of 20%,and the throttle is such that the engine cycles 10 times a second,the heat that is expelled in one cycle is
(Multiple Choice)
4.7/5  (30)
(30)
For a gas of N identical molecules of molecular volume Vm in total volume V at temperature T,the number of ways of locating the N molecules in the volume is
(Multiple Choice)
4.7/5  (34)
(34)
A Carnot cycle,operating as a heat engine,consists,in the order given,of
(Multiple Choice)
4.8/5  (35)
(35)
An engine is designed to obtain energy from the temperature gradient of the ocean.What is the thermodynamic efficiency of such an engine if the temperature of the surface of the water is 59°F (15°C)and the temperature well below the surface is 41°F (5°C)?
(Multiple Choice)
4.7/5  (32)
(32)
Find the change in entropy (in J/K)when 5.00 moles of an ideal gas undergo a free expansion from an initial volume of 20 cm3 to a final volume of 100 cm3.
(Multiple Choice)
4.8/5  (43)
(43)
The change in entropy of 1.00 kg of water that is heated from 50°C to 100°C is (in cal/K)
(Multiple Choice)
4.7/5  (31)
(31)
The reason that we can calculate the change in entropy of a system is that
(Multiple Choice)
4.7/5  (32)
(32)
A gasoline engine absorbs 2 500 J of heat and performs 1 000 J of mechanical work in each cycle.The amount of heat expelled in each cycle is
(Multiple Choice)
4.8/5  (37)
(37)
On a cold day,a heat pump absorbs heat from the outside air at 14°F (−10°C)and transfers it into a home at a temperature of 86°F (30°C).Determine the maximum κ of the heat pump.
(Multiple Choice)
4.9/5  (38)
(38)
A new electric power plant has an efficiency of 42%.For every 100 barrels of oil needed to run the turbine,how many are essentially lost as waste heat (in barrels of oil)to the environment?
(Multiple Choice)
4.9/5  (24)
(24)
An adiabatic free expansion of a gas in a thermally isolated container is not reversible because
(Multiple Choice)
4.9/5  (33)
(33)
Showing 1 - 20 of 55
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)