Exam 43: Molecules and Solids
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion46 Questions
Exam 17: Sound Waves48 Questions
Exam 18: Superposition and Standing Waves60 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields83 Questions
Exam 24: Gausss Law80 Questions
Exam 25: Electric Potential97 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field105 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 36: Image Formation46 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure50 Questions
Exam 45: Applications of Nuclear Physics40 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
An LED emits light of wavelength 600 nm.What is its band gap?
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
D
The diagram below shows the distance between the nuclei,pA and pB,and the electrons,e1 and e2,in a hydrogen molecule.We would expect the electrostatic potential energy of this molecule to be 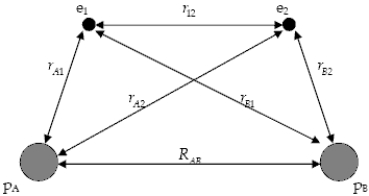
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
E
When a molecule jumps from a rotational energy level characterized by the rotational quantum number J to one characterized by J − 1,the difference in energy of levels J and J − 1,EJ − EJ − 1,is
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
D
The rotation spectrum of the HCl molecule suggests a photon in the far infrared (around 5.0 × 10−6 m)can excite the first rotational level.From this data,the moment of inertia of the HCl molecule (in kg ⋅ m2)is
(Multiple Choice)
4.9/5  (30)
(30)
What is the energy of the first rotational state of the hydrogen (H2)molecule? The separation between the protons is 10−10 m and the mass of each proton is 1.67 × 10−27 kg.(h = 6.626 × 10−34 J ⋅ s and 1 eV = 1.6 × 10−19 J. )
(Short Answer)
4.8/5  (40)
(40)
The Fermi temperature of copper is 80 000 K.The corresponding Fermi energy (in eV)is
(Multiple Choice)
4.9/5  (27)
(27)
An experiment determines that there are 49 allowed rotational energies for a diatomic molecule whose moment of inertia is 2 × 10−46 kg ⋅ m2.The maximum rotational kinetic energy (in eV)is
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following refer to the basic categories associated with the energy of a single molecule in a gaseous phase?
(Multiple Choice)
4.8/5  (32)
(32)
To find the number of electrons per unit volume with energy between E and E + dE in a metal we must multiply the number of allowed states per unit volume with energy E by
(Multiple Choice)
4.7/5  (33)
(33)
Solid argon has a density of 1650 kg/m3.The atomic weight of argon is 40.0.Assuming each atom occupies a cubical volume,what is the distance between the argon atoms?
(Short Answer)
4.8/5  (30)
(30)
When a molecule jumps from a rotational energy level characterized by the rotational quantum number J to one characterized by J + 1,the change in energy,EJ + 1 − EJ,is
(Multiple Choice)
4.7/5  (32)
(32)
A diatomic molecule consists of two point masses,m1 and m2,separated by a distance r.Find the moment of inertia through the center of mass about an axis perpendicular to the molecular axis.
(Multiple Choice)
4.8/5  (45)
(45)
The frequency of a microwave absorbed by a molecule when changing from the J = 3 to J = 4 rotation energy state is 4.61 × 1011 Hz.The moment of inertia of the molecule (in kg ⋅ m2)is
(Multiple Choice)
5.0/5  (37)
(37)
Assume the angular momentum of a diatomic molecule is quantized according to the relation 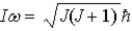 )What are the allowed rotational kinetic energies?
)What are the allowed rotational kinetic energies?
(Multiple Choice)
4.9/5  (34)
(34)
Ellis and Randy are looking at a molecular absorption spectrum.The spectral lines appear to fall into two groups with a gap in the middle.Ellis says that this must be an absorption spectrum for transitions between the v = 0 and v = 1 vibrational states of a diatomic molecule.Randy says the gap in the middle must occur because a ΔJ = 0 transition is forbidden.Which one,if either,is correct,and why?
(Multiple Choice)
4.8/5  (39)
(39)
In the hydrogen molecule,H2,the separation between the protons is 10−10 m.If the molecule is in its first rotational energy state,what is the angular velocity of the molecule about its center of mass?
(Short Answer)
4.8/5  (41)
(41)
A diatomic molecule consists of two point masses,m1 and m2,separated by a distance r.If x is the distance from m1 to the center of mass,find the moment of inertia in terms of x about an axis parallel to the molecular axis through the center of mass.
(Multiple Choice)
4.8/5  (43)
(43)
The energy gap for germanium is 0.670 eV at room temperature.What wavelength must a photon have (in nm)to excite the electron to the conduction band?
(Multiple Choice)
4.8/5  (38)
(38)
The fundamental frequency of CO is 6.42 × 1013 Hz.If the atomic masses are 12 u and 16 u (1 u = 1.66 × 10−27 kg),find the force constant (in N/m)for the diatomic molecule.
(Multiple Choice)
4.9/5  (29)
(29)
Showing 1 - 20 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)