Exam 13: The Atomic Nucleus and Radioactivity
Exam 1: Patterns of Motion and Equilibrium94 Questions
Exam 2: Newtons Laws of Motion109 Questions
Exam 3: Momentum and Energy128 Questions
Exam 4: Gravity, Projectiles, and Satellites114 Questions
Exam 5: Fluid Mechanics120 Questions
Exam 6: Thermal Energy and Thermodynamics100 Questions
Exam 7: Heat Transfer and Change of Phase115 Questions
Exam 8: Static and Current Electricity144 Questions
Exam 9: Magnetism and Electromagnetic Induction105 Questions
Exam 10: Waves and Sound120 Questions
Exam 11: Light146 Questions
Exam 12: Atoms and the Periodic Table128 Questions
Exam 13: The Atomic Nucleus and Radioactivity124 Questions
Exam 14: Elements of Chemistry49 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React118 Questions
Exam 18: Two Classes of Chemical Reactions182 Questions
Exam 19: Organic Compounds98 Questions
Exam 20: Rocks and Minerals170 Questions
Exam 21: Plate Tectonics and Earths Interior175 Questions
Exam 22: Shaping Earths Surface175 Questions
Exam 23: Geologic Timereading the Rock Record145 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects172 Questions
Exam 25: Driving Forces of Weather145 Questions
Exam 26: The Solar System87 Questions
Exam 27: Stars and Galaxies84 Questions
Exam 28: The Structure of Space and Time55 Questions
Exam 29: Prologue: the Nature of Science22 Questions
Select questions type
How many alpha particles are emitted in the series of radioactive decay events from a U-238 nucleus to a Pb-206 nucleus?
(Multiple Choice)
4.8/5  (35)
(35)
Why does plutonium not occur in appreciable amounts in natural ore deposits?
(Multiple Choice)
4.9/5  (37)
(37)
Which process would release energy from gold, fission or fusion? From carbon?
(Multiple Choice)
4.9/5  (38)
(38)
Elements above uranium in the periodic table do not exist in any appreciable amounts in nature because they have short half-lives. Yet there are several elements below uranium in atomic number with equally short half-lives that do exist in appreciable amounts in nature. How can you account for this?
(Multiple Choice)
4.8/5  (33)
(33)
The element uranium (U, atomic no. = 92)often has 146 (or more)neutrons but it will still undergo radioactive decay. Which statement might best describe why?
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following statements about carbon-14 dating is true?
(Multiple Choice)
5.0/5  (38)
(38)
Just after an alpha particle leaves the nucleus, would you expect it to speed up? Why?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following statements about fusion is the most accurate?
(Multiple Choice)
4.8/5  (39)
(39)
In the 19th century the famous physicist Lord Kelvin estimated the age of Earth to be much much less than its present estimate. What information did Kelvin not have to account for his error?
(Multiple Choice)
4.9/5  (34)
(34)
Light nuclei can be split. For example, a deuteron, which is a proton-neutron combination, can split into a separate proton and separate neutron. Does such a process yield energy or cost energy? Why?
(Multiple Choice)
4.8/5  (43)
(43)
If uranium were to split into three segments of equal size instead of two, would more energy or less energy be released?
(Multiple Choice)
4.9/5  (26)
(26)
Which of the following nuclear equations correctly describes beta emission?
(Multiple Choice)
4.9/5  (35)
(35)
Why would you expect alpha particles to be less able to penetrate materials than beta particles of the same kinetic energy?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following nuclear equations correctly describes alpha emission?
(Multiple Choice)
4.7/5  (30)
(30)
Explain how radioactive decay has always warmed the earth from the inside, and nuclear fusion has always warmed the earth from the outside.
(Multiple Choice)
4.8/5  (35)
(35)
If a nucleus of 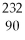 Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
(Multiple Choice)
4.8/5  (37)
(37)
Alpha and beta rays are deflected in opposite directions in a magnetic field because
(Multiple Choice)
4.8/5  (38)
(38)
Which type of radiation-alpha, beta, or gamma-results in the least change in atomic number?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 41 - 60 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)