Exam 13: The Atomic Nucleus and Radioactivity
Exam 1: Patterns of Motion and Equilibrium94 Questions
Exam 2: Newtons Laws of Motion109 Questions
Exam 3: Momentum and Energy128 Questions
Exam 4: Gravity, Projectiles, and Satellites114 Questions
Exam 5: Fluid Mechanics120 Questions
Exam 6: Thermal Energy and Thermodynamics100 Questions
Exam 7: Heat Transfer and Change of Phase115 Questions
Exam 8: Static and Current Electricity144 Questions
Exam 9: Magnetism and Electromagnetic Induction105 Questions
Exam 10: Waves and Sound120 Questions
Exam 11: Light146 Questions
Exam 12: Atoms and the Periodic Table128 Questions
Exam 13: The Atomic Nucleus and Radioactivity124 Questions
Exam 14: Elements of Chemistry49 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React118 Questions
Exam 18: Two Classes of Chemical Reactions182 Questions
Exam 19: Organic Compounds98 Questions
Exam 20: Rocks and Minerals170 Questions
Exam 21: Plate Tectonics and Earths Interior175 Questions
Exam 22: Shaping Earths Surface175 Questions
Exam 23: Geologic Timereading the Rock Record145 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects172 Questions
Exam 25: Driving Forces of Weather145 Questions
Exam 26: The Solar System87 Questions
Exam 27: Stars and Galaxies84 Questions
Exam 28: The Structure of Space and Time55 Questions
Exam 29: Prologue: the Nature of Science22 Questions
Select questions type
Why doesn't uranium ore spontaneously undergo a chain reaction?
(Multiple Choice)
4.7/5  (38)
(38)
Why might the following nuclear reaction not be very good for energy production in a fission reactor? 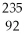 U →
U → 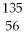 Ba +
Ba + 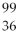 Kr + 1 neutron
Kr + 1 neutron
(Multiple Choice)
4.9/5  (41)
(41)
If a nucleus of 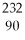 Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
(Multiple Choice)
4.9/5  (43)
(43)
What does Einstein's energy equation (E = mc2)say about the energy that is derived from nuclear fission reactions?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following nuclear equations correctly describes beta emission?
(Multiple Choice)
4.8/5  (44)
(44)
What property of half-lives makes radioactive material so problematic?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following sources of radiation provides most of our yearly exposure?
(Multiple Choice)
4.8/5  (37)
(37)
The age of the Dead Sea Scrolls was found by carbon-14 dating. Could this technique have worked if they were carved in stone tablets? Explain.
(Multiple Choice)
4.7/5  (41)
(41)
What are some of the optimistic worldwide changes that may follow the advent of successful fusion reactors?
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following statements best describes the strong nuclear force?
(Multiple Choice)
4.8/5  (37)
(37)
If three samples have the half-lives listed below, which sample would remain radioactive the longest?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following provides the minimum amount of protection you need to block the following form of radiation? beta
(Multiple Choice)
4.7/5  (32)
(32)
Why is carbon better than lead as a moderator in nuclear reactors?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following provides the minimum amount of protection you need to block the following form of radiation? alpha
(Multiple Choice)
4.8/5  (30)
(30)
When the isotope bismuth-213 emits an alpha particle, what new element results?
(Multiple Choice)
4.7/5  (44)
(44)
The isotope Cesium-137, which has a half-life of 30 years, is a product of nuclear power plants. How long will it take for this isotope to decay to about one-sixteenth its original amount?
(Multiple Choice)
4.8/5  (40)
(40)
Does the average distance that a neutron travels through fissionable material before escaping increase or decrease when two pieces of fissionable material are assembled into one piece? Does this assembly increase or decrease the probability of an explosion?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following nuclear equations correctly describes alpha emission?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 101 - 120 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)