Exam 14: Introduction to Metabolism
Exam 1: Introduction to the Chemistry of Life56 Questions
Exam 2: Water59 Questions
Exam 3: Nucleotides, Nucleic Acids, and Genetic Information68 Questions
Exam 4: Amino Acids68 Questions
Exam 5: Proteins: Primary Structure68 Questions
Exam 6: Proteins: Three-Dimensional Structure60 Questions
Exam 7: Protein Function Part I: Myoglobin and Hemoglobin74 Questions
Exam 8: Carbohydrates66 Questions
Exam 9: Lipids and Biological Membranes59 Questions
Exam 10: Membrane Transport50 Questions
Exam 11: Enzyme Catalysis53 Questions
Exam 12: Enzyme Kinetics, Inhibition and Control55 Questions
Exam 13: Biochemical Signaling50 Questions
Exam 14: Introduction to Metabolism52 Questions
Exam 15: Glycogen Metabolism and Gluconeogenesis50 Questions
Exam 16: Glucose Catabolism50 Questions
Exam 17: Citric Acid Cycle50 Questions
Exam 18: Electron Transport and Oxidative Phosphorylation50 Questions
Exam 19: Photosynthesis50 Questions
Exam 20: Lipid Metabolism51 Questions
Exam 21: Amino Acid Metabolism50 Questions
Exam 22: Mammalian Fuel Metabolism: Integration and Regulation50 Questions
Exam 23: Nucleotide Metabolism50 Questions
Exam 24: Nucleic Acid Structure50 Questions
Exam 25: Dna Replication, Repair and Recombination50 Questions
Exam 26: Transcription and Rna Processing50 Questions
Exam 27: Translation49 Questions
Exam 28: Regulation of Gene Expression50 Questions
Select questions type
In redox half-reactions, a more positive standard reduction potential means
I.the oxidized form has a higher affinity for electrons.
II.the oxidized form has a lower affinity for electrons.
III.the reduced form has a higher affinity for electrons.
IV.the greater the tendency for the oxidized form to accept electrons.
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following are factors that contribute to the large negative standard free energy change for the reaction shown below?
ATP ADP + Pi
(Multiple Choice)
4.9/5  (30)
(30)
The enzyme inorganic pyrophosphatase catalyzes the hydrolysis of bonds in ___.
(Multiple Choice)
4.9/5  (38)
(38)
Matching
-1,3-Bisphosphoglycerate is an example of a(n)______.
(Multiple Choice)
4.9/5  (41)
(41)
Matching
-A metabolic reaction resulting in the formation of FADH2 is an example of a(n)___ reaction.
(Multiple Choice)
4.8/5  (42)
(42)
Which of the choices correctly ranks the following compounds from lowest level of oxidation to highest level of oxidation? 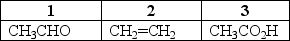
(Multiple Choice)
4.9/5  (29)
(29)
The ______ is equal to the rate of synthesis minus the rate of breakdown of the metabolic intermediates.
(Multiple Choice)
4.8/5  (42)
(42)
Matching
-The study of the complete set of proteins synthesized in the cell in response to changing conditions is called ______.
(Multiple Choice)
4.9/5  (35)
(35)
Matching
-The enzyme ______ catalyzes the transfer of a phosphoryl group from ATP to AMP.
(Multiple Choice)
4.9/5  (34)
(34)
Consider the following metabolic reaction:
3-Phosphoglycerate 2-Phosphoglycerate G°' = +4.40 kJ/mol
What is the G for this reaction when the concentration of 2-phosphoglycerate is 0.290 mM and the concentration of 3-phosphoglycerate is 2.90 mM at 37°C?
(Multiple Choice)
4.7/5  (39)
(39)
Consider the following metabolic reaction important in muscle and nerve cells:
ATP + creatine phosphocreatine + ADP G°' = +12.6 kJ/mol
Under intracellular conditions, the G for the reaction, which is catalyzed by the enzyme creatine kinase, is ~0 kJ/mol.From this information we can conclude that
(Multiple Choice)
4.7/5  (35)
(35)
Matching
-The enzyme ______ catalyzes the reaction, PPi 2 Pi.
(Multiple Choice)
4.9/5  (38)
(38)
Showing 41 - 52 of 52
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)