Exam 2: Particles of Matter
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
How would you describe the size of the following object? a blood cell
(Multiple Choice)
4.7/5  (44)
(44)
Humidity is a measure of the amount of water vapor in the atmosphere. Why is humidity always very low inside your kitchen freezer?
(Multiple Choice)
4.9/5  (30)
(30)
Which would have the most kinetic energy? The same mass of ________.
(Multiple Choice)
4.9/5  (38)
(38)
Each sphere in the diagrams below represents an atom. Joined spheres represent molecules. Assume the two boxes are at the same temperature. Which box contains a higher boiling point liquid? 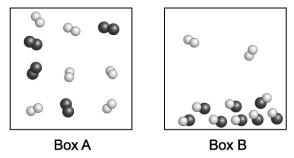
(Multiple Choice)
4.7/5  (36)
(36)
Showing 101 - 104 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)