Exam 2: Particles of Matter
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Why do scuba divers exhale air when they ascend to the surface after a dive?
(Multiple Choice)
4.8/5  (38)
(38)
Does a 2 kg solid iron brick have twice as much mass as a 1 kg solid block of wood? Twice as much volume?
(Multiple Choice)
4.8/5  (32)
(32)
Can an object have mass without having weight? Can it have weight without having mass?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following is something that is best described as having potential energy?
(Multiple Choice)
4.8/5  (34)
(34)
What volume of water would a 52.3-gram sample of pure gold displace? (Assume the density of pure gold equals 19.3 g/mL)
(Multiple Choice)
4.8/5  (32)
(32)
If the density of mercury is 13.6 g/mL and the density of lead is 11.3 g/mL, which has the larger volume: 1 g of mercury or 1 g of lead?
(Multiple Choice)
4.8/5  (35)
(35)
Which has stronger attractions among its submicroscopic particles: a solid at 25°C or a gas at 
(Multiple Choice)
4.9/5  (43)
(43)
Why is it not possible for the scanning probe microscope (SPM) to make images of the inside of an atom?
(Multiple Choice)
4.9/5  (38)
(38)
The following three boxes represent the number of submicroscopic particles within a given volume of a particular substance at different temperatures. Which box represents the greatest density? Which box represents the greatest temperature? 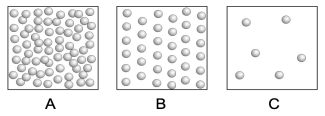
(Multiple Choice)
4.7/5  (40)
(40)
The diagram on the far left shows the moving particles of a gaseous material within a rigid container. Which of the three boxes on the right best represents this material upon the addition of heat? 
(Multiple Choice)
4.8/5  (36)
(36)
What physical quantities discussed in this chapter change most when a junked car is neatly crushed into a compact cube?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 21 - 40 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)