Exam 11: Using Energy
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a clockwise sense around the enclosed area, as shown on the figure, then the change of internal energy over the full cycle 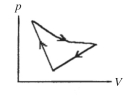
(Multiple Choice)
4.7/5  (31)
(31)
Oxygen condenses into a liquid at approximately 90 K. What temperature, in degrees Fahrenheit, does this correspond to?
(Multiple Choice)
4.9/5  (33)
(33)
A refrigerator has a coefficient of performance equal to 4.2. How much work must be done on the operating gas in the refrigerator in order to remove 250 J of heat from the interior compartment?
(Multiple Choice)
4.7/5  (36)
(36)
A refrigerator has a performance coefficient (COP) of 1.15, and it extracts 7.95 J of heat from the cold reservoir during each cycle.
(a) How much work is done on the gas in each cycle?
(b) How much heat is exhausted into the hot reservoir in each cycle?
(Short Answer)
5.0/5  (33)
(33)
If the efficiency of a Carnot engine were to be 100%, the heat sink would have to be
(Multiple Choice)
4.8/5  (28)
(28)
During each cycle, a heat engine takes in 4.0 J of heat, does 1.0 J of work, and expels 3.0 J of heat. What is its efficiency?
(Short Answer)
4.7/5  (40)
(40)
A sealed rigitd tank contains 30 moles of an ideal gas, at an initial temperature of The pressure of the gas is increased until the final pressure equals 1.40 times the initial pressure. The heat capacity at constant pressure of the gas is What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (35)
(35)
At room temperature, a typical person loses energy to the surroundings at the rate of 62 W. If this energy loss has to be made up by an equivalent food intake, how many kilocalories (food calories) does this person need to consume every day just to make up this heat loss? (1 cal = 4.186 J)
(Multiple Choice)
4.9/5  (42)
(42)
During each cycle of operation, a refrigerator absorbs 230 J of heat from the freezer and expels 356 J of heat to the room. How much work input is required in each cycle?
(Multiple Choice)
4.8/5  (33)
(33)
During an isothermal process, 5.0 J of heat is removed from an ideal gas. What is the change in internal (thermal) energy of the gas?
(Multiple Choice)
4.9/5  (29)
(29)
The work done on an ideal gas system in an isothermal process is -400 J. What is the change in internal (thermal) energy of the gas?
(Multiple Choice)
4.9/5  (37)
(37)
At what, if any, temperature are the numerical readings on the Fahrenheit and Celsius scales the same?
(Multiple Choice)
4.8/5  (33)
(33)
A reversible engine takes in heat at a rate of 555 W and exhausts heat at 482 W.
(a) How much power does it produce?
(b) What is its efficiency?
(c) If operated in reverse as a refrigerator, what would be its performance coefficient (COP)?
(Short Answer)
4.8/5  (40)
(40)
A heat engine absorbs 64 kcal of heat each cycle and exhausts 42 kcal.
(a) What is the efficiency of this engine?
(b) How much work does this engine do per cycle?
(Short Answer)
4.9/5  (30)
(30)
An ideal Carnot air conditioner operates between an indoor temperature of 20°C and an outdoor temperature of 39°C. How much energy does it use to remove 2000 J of heat from the interior of the house?
(Multiple Choice)
4.8/5  (34)
(34)
During an isochoric process, the internal (thermal) energy of a gas decreases by 50 J. How much heat is added to the gas during this process?
(Multiple Choice)
4.8/5  (33)
(33)
A heat engine having the maximum possible efficiency has an efficiency of 25% when operating between two heat reservoirs. If the temperature of the cold reservoir is 300 K, what is the temperature of the hot reservoir?
(Multiple Choice)
4.8/5  (36)
(36)
During each cycle, the compressor in a certain ideal Carnot refrigerator performs 480 J of work to remove 150 J of heat from the interior of the refrigerator. How much heat do the coils behind the refrigerator discharge into the kitchen each cycle?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 61 - 80 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)