Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
If the temperature of an ideal gas is increased from 20°C to 40°C, by what percent does the speed of the molecules increase?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
A
An aluminum rod 17.400 cm long at 20°C is heated to 100°C. What is its new length? Aluminum has a linear expansion coefficient of 25 × 10-6 K-1.
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
A
A 0.50 gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of The atomic mass of nitrogen is . What is the rms speed of the molecules? (The Boltzmann constant is 1.38 × 10-23 J/K, NA = 6.022 × 1023 molecules/mol.)
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
A
The coefficient of linear expansion for aluminum is 1.8 × 10-6 K-1. What is its coefficient of volume expansion?
(Multiple Choice)
4.9/5  (37)
(37)
Oxygen molecules are 16 times more massive than hydrogen molecules. At a given temperature, the average molecular kinetic energy of oxygen molecules, compared to that of hydrogen molecules,
(Multiple Choice)
4.8/5  (31)
(31)
A mole of diatomic oxygen molecules and a mole of diatomic nitrogen molecules are at STP. Which statements are true about these molecules? (There could be more than one correct choice.)
(Multiple Choice)
4.8/5  (31)
(31)
Two metal rods, one silver and the other copper, are both immersed at one end in a steam chamber at a temperature of 100°C. The other end of each one is in an ice water bath at 0°C. The rods are 5.0 cm long and have a square cross-section that is 2.0 cm on a side. No heat is exchanged between the rods and the surroundings, except at the ends. How much total heat flows through the two rods each minute? The thermal conductivity of silver is 417 W/m ∙ K, and that of copper is 395 W/m ∙ K.
(Multiple Choice)
4.9/5  (39)
(39)
A .20-kg ice cube at 0.0°C has sufficient heat added to it to cause total melting, and the resulting water is heated to How much heat is added? For water LF = 334,000 J/kg, LV = 2.256 × 106 J/kg, the c = 4.186 x 103 J/kg ∙ C.
(Multiple Choice)
4.9/5  (32)
(32)
A gas expands from an initial volume of 0.040 m3 to a final volume of 0.085 m3 while its pressure increases linearly with the volume (so that the process follows a straight-line path in a pV diagram) from 110 kPa to 225 kPa. How much work is done by the gas during this expansion?
(Multiple Choice)
4.9/5  (37)
(37)
On his honeymoon, James Joule attempted to explore the relationships between various forms of energy by measuring the rise of temperature of water which had fallen down a waterfall on Mount Blanc. What maximum temperature rise would one expect for a waterfall with a vertical drop of 20 m? The specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (35)
(35)
A 920-g empty iron kettle is put on a stove. How much heat in joules must it absorb to raise its temperature form to The specific heat for iron is 113 cal/kg ∙ C°, and 1 cal = 4.186 J.
(Multiple Choice)
4.9/5  (32)
(32)
What is the net power radiated by a little animal with a surface area of 0.075 m2 if his emissivity is 0.75, his skin temperature is 315 K, and he is in a room with a temperature of 290 K? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.8/5  (34)
(34)
If you add 1.33 MJ of heat to 500 g of water at 50°C in a sealed container, what is the final temperature of the steam? The latent heat of vaporization of water is 22.6 × 105 J/kg, the specific heat of steam is 2010 J/kg ∙ K, and the specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (31)
(31)
A camper is about to drink his morning coffee. He pours 400 grams of coffee, initially at 75°C into a 250-g aluminum cup, initially at 16°C. What is the equilibrium temperature of the coffee-cup system, assuming no heat is lost to the surroundings? The specific heat of aluminum is 900 J/kg ∙ K, and the specific heat of coffee is essentially the same as that of water, which is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (29)
(29)
The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container. The temperature of state 1 is 59°C, the atomic mass of the nitrogen atom is 14 g/mol, and R = 8.31 J/mol ∙ K. What are (a) pressure p1 and (b) temperature T2? 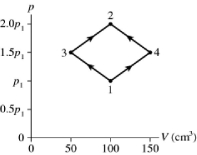
(Multiple Choice)
4.7/5  (30)
(30)
The coefficient of linear expansion of copper is 17 × 10-6 K-1 and that of steel is 12 × 10-6 K-1. At 12°C a steel rod has a diameter of 2.540 cm and a copper pipe has a diameter of 2.536 cm. If they are heated together to a higher temperature, which one of the following quantities is closest to the common temperature at which the steel rod will fit snugly in the copper pipe?
(Multiple Choice)
4.8/5  (33)
(33)
If 150 kcal of heat raises the temperature of 2.0 kg of a material by 400 F°, what is the specific heat capacity of the material?
(Multiple Choice)
4.8/5  (35)
(35)
A hole in a brass plate has a diameter of 1.200 cm at 20°C. What is the diameter of the hole when the plate is heated to 220°C? The coefficient of linear thermal expansion for brass is 19 × 10-6 K-1.
(Multiple Choice)
4.8/5  (40)
(40)
A 5.3 L flask of ideal neon gas (which is monatomic) is at a pressure of 6.0 atm and a temperature of The atomic mass of neon is 20.2 g/mol. What is the mass of the neon gas in the flask. (R = 8.31 J/mol ∙ K, 1 atm = 101 kPa, NA = 6.022 × 1023 molecules/mol)
(Multiple Choice)
4.7/5  (27)
(27)
A 90-g aluminum calorimeter contains 390 g of water at an equilibrium temperature of A piece of metal, initially at is added to the calorimeter. The final temperature at equilibrium is 32° C. Assume there is no external heat exchange. The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum) and 4190 J/kg ∙ K (water). What is the specific heat capacity of the 160-g piece of metal?
(Multiple Choice)
4.8/5  (21)
(21)
Showing 1 - 20 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)