Exam 11: Using Energy
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
An ideal Carnot engine is operated as a heat pump to heat a room in the winter. The heat pump delivers heat to the room at the rate of 47 kJ per second and maintains the room at a temperature of 293 K when the outside temperature is 237 K. The power requirement to run the heat pump under these operating conditions is closest to
(Multiple Choice)
4.9/5  (44)
(44)
A heat engine absorbs 85.6 kJ of heat each cycle and exhausts 61.8 kJ.
(a) What is the efficiency of the engine?
(b) How much work does it do each cycle?
(Short Answer)
4.9/5  (29)
(29)
A real (non-Carnot) heat engine, operating between heat reservoirs at temperatures of 450 K and 270 K, performs 3.3 kJ of net work, and rejects 8.2 kJ of heat in a single cycle.
(a) What is the thermal efficiency of this heat engine?
(b) What is the maximum efficiency it could possibly have?
(Short Answer)
4.8/5  (37)
(37)
A compression, at a constant pressure of 140 kPa, is performed on 4.0 moles of an ideal monatomic gas for which CV = 3/2 R. The compression reduces the volume of the gas from to What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (28)
(28)
A cylinder contains 1.50 moles of an ideal monatomic gas that is initially at a temperature of 317 K. If the gas gains 2730 J of heat and performs 780 J of work, what is its final temperature? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (30)
(30)
An expansion process on an ideal diatomic ideal gas for which CV = 5/2 R has a linear path between the initial and final coordinates on a pV diagram. The coordinates of the initial state are: the pressure is the volume is and the temperature is The final pressure is and the final temperature is What is the change in the internal (thermal) energy of the gas, during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
5.0/5  (32)
(32)
An ideal gas undergoes the process a→b→c→a shown in the pV diagram. The heat gained by the gas in process a→b is 546 J, while in process the gas loses 62.0 J of heat. In process a→b the gas performs of work, while in process c→a 223 J of work is done on the gas. How much heat is gained by the gas in process c→a? 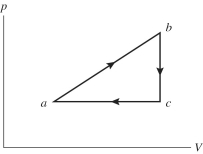
(Multiple Choice)
4.8/5  (35)
(35)
A heat pump with a performance coefficient (COP) of 4.9 absorbs heat from the atmosphere at a rate of At what rate is work being done to run this heat pump?
(Multiple Choice)
4.9/5  (44)
(44)
What is absolute zero on the (a) Celsius scale and (b) on the Fahrenheit scale?
(Short Answer)
4.7/5  (35)
(35)
An expandable container holds 2.30 mole of helium, He, gas with an initial pressure of 770 kPa and an initial volume of 2.10 L. The gas expands isothermally to a final pressure of 350 kPa. How much heat is gained by the gas during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (33)
(33)
A nuclear power plant has an actual efficiency of 33%. If of energy are released from fission, how much electric power does the power plant produce?
(Multiple Choice)
4.8/5  (32)
(32)
A coal-fired plant generates 600 MW of electric power. The plant uses 4.8 × 106 kg of coal each day, and the heat of combustion of coal is 3.3 × 107 J/kg. The steam that drives the turbines is at a temperature of 300°C, and the exhaust water is at 37°C.
(a) What is the overall efficiency of the plant for generating electric power?
(b) How much thermal energy is exhausted each day?
(c) Using the same heat reservoirs, what is the maximum possible efficiency for a heat engine?
(Short Answer)
4.8/5  (32)
(32)
A 40.0-L container is divided into two equal parts by a rubber membrane. One half of the container has 1.50 moles of an ideal monatomic gas at 250 K, and the other half is a vacuum. The container is well insulated, so there is no exchange of heat with the surroundings. The membrane breaks, and eventually the gas reaches a new equilibrium condition occupying the entire volume. What is the final temperature of the gas?
(Multiple Choice)
4.8/5  (36)
(36)
An ideal Carnot heat engine operates between reservoirs at 1740 K and In each cycle, 260 J of heat energy is rejected to the low temperature reservoir. In each cycle, how much mechanical work W is performed by the engine?
(Short Answer)
4.8/5  (33)
(33)
A heat engine with an efficiency of 30% performs 2500 J of work. How much heat is discharged to the lower temperature reservoir?
(Multiple Choice)
4.9/5  (39)
(39)
During each cycle, a refrigerator removes 20.0 kJ of heat from the freezing compartment and ejects 24.0 kJ into a room.
(a) How much work per cycle is required each cycle to run this refrigerator?
(b) What is the coefficient of performance of this refrigerator?
(Short Answer)
4.9/5  (35)
(35)
A heat engine has an efficiency of 31.4% and receives 8.72 kJ of heat per cycle.
(a) How much work does it perform in each cycle?
(b) How much heat does it exhaust in each cycle?
(Short Answer)
4.9/5  (29)
(29)
Showing 41 - 60 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)