Exam 9: Thermodynamics: The Second and Third Laws
Exam 1: The Quantum World100 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids95 Questions
Exam 7: Inorganic Materials100 Questions
Exam 8: Thermodynamics: The First Law94 Questions
Exam 9: Thermodynamics: The Second and Third Laws95 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria94 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics93 Questions
Exam 16: The Elements: the Main Group Elements189 Questions
Exam 17: The Elements: The D Block94 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I94 Questions
Exam 20: Organic Chemistry II95 Questions
Select questions type
Use the Boltzmann formula to calculate the entropy at T = 0 of 1.00 mol chlorobenzene,C6H5Cl,where each molecule can be oriented in any of six ways.
(Multiple Choice)
4.8/5  (39)
(39)
The entropy of vaporization of a substance is always larger than its entropy of fusion.
(True/False)
4.9/5  (23)
(23)
The hydrolysis of ATP to ADP is spontaneous.However,the reaction of glucose with monohydrogen phosphate,the first step in the oxidation of glucose,is not spontaneous.Determine whether the coupled reactions are spontaneous.
(Essay)
4.9/5  (38)
(38)
For the reaction
2SO3(g) 2SO2(g)+ O2(g)
Hr° = +198 kJ.mol-1 and Sr° = 190 J.K-1.mol-1 at 298 K.The equilibrium constant for this reaction will be greater than 1 at
(Multiple Choice)
4.9/5  (40)
(40)
Use tabulated thermodynamic data to calculate the concentration of CO2(aq)in equilibrium with an external pressure of 2.50 atm CO2(g)at 298 K.
(Short Answer)
4.9/5  (30)
(30)
The experimental value of the molar entropy of 1 mol NO at 0 K is about 5 J∙K-1.We can conclude that in the crystal the molecules of NO are arranged randomly.
(True/False)
4.8/5  (40)
(40)
For He(g,10 atm) He(g,1 atm),is the entropy change positive or negative?
(Short Answer)
4.8/5  (42)
(42)
Calculate the standard entropy of condensation of chloroform at its boiling point,335 K.The standard molar enthalpy of vaporization of chloroform at its boiling point is 31.4 J.K-1.mol-1 .
(Multiple Choice)
4.9/5  (34)
(34)
Consider the following compounds and their standard free energies of formation: 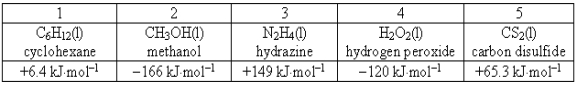 Which of these liquids is (are)thermodynamically stable?
Which of these liquids is (are)thermodynamically stable?
(Multiple Choice)
4.9/5  (47)
(47)
Consider the following compounds and their standard free energies of formation:  Which of these liquids is (are)thermodynamically unstable?
Which of these liquids is (are)thermodynamically unstable?
(Multiple Choice)
4.9/5  (37)
(37)
For the reaction
2C(s)+ 2H2(g) C2H4(g)
Hr = +52.3 kJ.mol-1 and Sr = -53.07 J.K-1.mol-1 at 298 K.The reverse reaction will be spontaneous at
(Multiple Choice)
5.0/5  (44)
(44)
Calculate Ssurr° at 298 K for the reaction 6C(s)+ 3H2(g) C6H6(l)
Hr° = +49.0 J.K-1.mol-1 , Sr° = -253 J.K-1.mol-1
(Multiple Choice)
4.8/5  (34)
(34)
Use Trouton's constant to estimate the enthalpy of vaporization of diethyl ether,which boils at 309 K.
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the standard entropy of vaporization of ethanol at its boiling point,352 K.The standard molar enthalpy of vaporization of ethanol at its boiling point is 40.5 J.K-1.mol-1 .
(Multiple Choice)
4.8/5  (45)
(45)
Consider the reaction Cl2(g) 2Cl(g)
Which of the following statement regarding this reaction is true?
(Multiple Choice)
4.9/5  (31)
(31)
The entropy of fusion of water is +22.0 J.K-1.mol-1 and the enthalpy of fusion of water is +6.01 J.K-1.mol-1 at 0 C.At 0 C, Stotal for the melting of ice is
(Multiple Choice)
4.9/5  (34)
(34)
Calculate G for the process
He(g,1 atm,298 K) He(g,10 atm,298 K)
(Short Answer)
4.9/5  (38)
(38)
Draw a graph of the molar Gibbs free energy versus temperature for H2O(l)and H2O(g).
(a)Explain the slopes of the 2 lines.
(b)At low temperatures,which phase is most stable?
(c)At high temperatures,which phase is more stable?
(Essay)
4.8/5  (28)
(28)
Calculate Stotal for the isothermal irreversible free expansion of 1.00 mol of ideal gas from 8.00 L to 20.00 L at 298 K.
(Multiple Choice)
4.9/5  (29)
(29)
The change in molar entropy for vaporization of all liquids is about the same.
(True/False)
4.9/5  (40)
(40)
Showing 41 - 60 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)