Exam 9: Thermodynamics: The Second and Third Laws
Exam 1: The Quantum World100 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids95 Questions
Exam 7: Inorganic Materials100 Questions
Exam 8: Thermodynamics: The First Law94 Questions
Exam 9: Thermodynamics: The Second and Third Laws95 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria94 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics93 Questions
Exam 16: The Elements: the Main Group Elements189 Questions
Exam 17: The Elements: The D Block94 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I94 Questions
Exam 20: Organic Chemistry II95 Questions
Select questions type
For a given transfer of energy,a greater change in disorder occurs when the temperature is high.
(True/False)
5.0/5  (45)
(45)
When calculating the entropy change as a result of transferring heat reversibly to or from a system,the temperature must be constant.
(True/False)
4.9/5  (47)
(47)
Calculate the entropy of vaporization of water at 25 C and 1 bar.The molar heat capacities of the liquid and gas are 75 and 34 J·K-1·mol-1,respectively.The molar enthalpy of vaporization of water at its normal boiling point is 40.7 kJ·mol-1.
(Short Answer)
4.7/5  (42)
(42)
For CO2(g) CO2(aq),is the entropy change positive or negative?
(Short Answer)
4.8/5  (27)
(27)
Sketch a plot of the molar entropy of oxygen gas from 0 K to 200 K.The normal melting and boiling points of oxygen are 55 K and 90 K,respectively.
(Essay)
4.9/5  (38)
(38)
For the reaction 2SO3(g) 2SO2(g)+ O2(g)
Hr = +198 kJ.mol-1 at 298 K.Which statement is true for this reaction?
(Multiple Choice)
4.8/5  (41)
(41)
Calculate the standard entropy of fusion of ethanol at its melting point,159 K.The standard molar enthalpy of fusion of ethanol at its melting point is 5.02 J.K-1.mol-1
(Multiple Choice)
4.8/5  (46)
(46)
The energy levels of two particle in a box systems are given below: 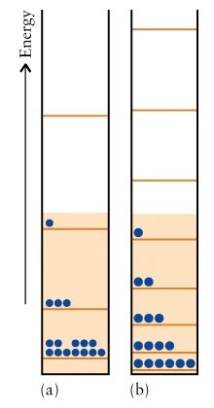 Which molecule,N2,or F2,is better represented by (b)?
Which molecule,N2,or F2,is better represented by (b)?
(Short Answer)
4.8/5  (40)
(40)
Use Trouton's constant to estimate the enthalpy of condensation of diethyl ether,which boils at 309 K.
(Multiple Choice)
4.8/5  (31)
(31)
Calculate Ssurr° at 298 K for the reaction H2(g)+ F2(g) 2HF(g)
Hr° = -546 kJ.mol-1, Sr° = +14.1 J.K-1.mol-1
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following would probably have a positive S value?
(Multiple Choice)
4.9/5  (37)
(37)
The molar entropy of silver at 298 K is equal to the area under the curve obtained by plotting (from T = 0 to T = 298 K)
(Multiple Choice)
4.7/5  (34)
(34)
The reaction N2(g)+ 3H2(g) 2NH3(g)is exothermic.This reaction will be spontaneous at all temperatures.
(True/False)
5.0/5  (42)
(42)
Predict the sign of the molar Gibbs free energy for the process H2O(s)→ H2O(l)at 1 atm and 1oC,0oC,and -1oC,respectively.
(Multiple Choice)
4.7/5  (39)
(39)
When barium hydroxide is dissolved in water,the temperature of the solution increases.Which of the following statements regarding this reaction is true?
(Multiple Choice)
4.7/5  (33)
(33)
The energy levels of two particle in a box systems are 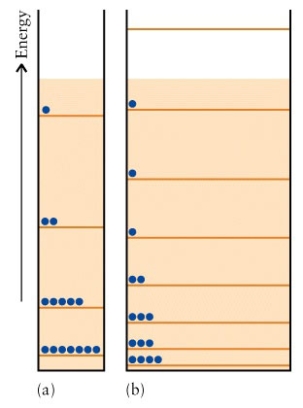 Which system has the higher entropy?
Which system has the higher entropy?
(Short Answer)
4.9/5  (40)
(40)
Iodine sublimes at room temperature.The reaction free energy,enthalpy,and entropy for this reaction,respectively,are
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following has the smallest molar entropy at 298 K?
(Multiple Choice)
4.9/5  (32)
(32)
Water slowly evaporates at 25oC.Which of the following statements regarding this reaction is true?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 61 - 80 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)