Exam 4: Chemical Quantities and Aqueous Reactions
Exam 1: Matter, measurement, and Problem Solving170 Questions
Exam 2: Atoms and Elements157 Questions
Exam 3: Molecules,compounds,and Chemical Equations175 Questions
Exam 4: Chemical Quantities and Aqueous Reactions239 Questions
Exam 5: Gases182 Questions
Exam 6: Thermochemistry143 Questions
Exam 7: The Quantum-Mechanical Model of the Atom134 Questions
Exam 8: Periodic Properties of the Elements147 Questions
Exam 9: Chemical Bonding I: Lewis Theory166 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,144 Questions
Exam 11: Liquids,solids,and Intermolecular Forces128 Questions
Exam 12: Solids and Modern Materials81 Questions
Exam 13: Solutions157 Questions
Exam 14: Chemical Kinetics154 Questions
Exam 15: Chemical Equilibrium141 Questions
Exam 16: Acids and Bases160 Questions
Exam 17: Aqueous Ionic Equilibrium187 Questions
Exam 18: Free Energy and Thermodynamics130 Questions
Exam 19: Electrochemistry151 Questions
Exam 20: Radioactivity and Nuclear Chemistry135 Questions
Exam 21: Organic Chemistry104 Questions
Exam 22: Biochemistry68 Questions
Exam 23: Chemistry of the Nonmetals66 Questions
Exam 24: Metals and Metallurgy60 Questions
Exam 25: Transition Metals and Coordination Compounds73 Questions
Select questions type
Give the complete ionic equation for the reaction (if any)that occurs when aqueous solutions of Na2CO3 and HCl are mixed.
(Multiple Choice)
4.8/5  (33)
(33)
Give the complete ionic equation for the reaction (if any)that occurs when aqueous solutions of K2S and Fe(NO3)2 are mixed.
(Multiple Choice)
4.8/5  (25)
(25)
How many grams of NaCl are required to make 250.00 mL of a 3.000 M solution?
(Multiple Choice)
4.9/5  (42)
(42)
Balance the chemical equation given below,and determine the number of milliliters of 0.00300 M phosphoric acid required to neutralize 35.00 mL of 0.00150 M calcium hydroxide. _____ Ca(OH)2(aq)+ _____ H3PO4(aq)→ _____ Ca3(PO4)2(aq)+ _____ H2O(l)
(Multiple Choice)
4.8/5  (35)
(35)
What is the molar concentration of phosphate ions in a 0.350 M Li3PO4 solution?
(Multiple Choice)
4.8/5  (33)
(33)
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2 Mg(s)+ 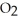 (g)→ 2 MgO(s)
When 3.50 g of magnesium burns,the theoretical yield of magnesium oxide is ________ g.
(g)→ 2 MgO(s)
When 3.50 g of magnesium burns,the theoretical yield of magnesium oxide is ________ g.
(Multiple Choice)
4.8/5  (34)
(34)
Identify the oxidation state of Mg in Mg(s). Mg(s)+ 2 HCl(aq)→ MgCl2(aq)+ H2(g)
(Multiple Choice)
4.8/5  (35)
(35)
How many grams of C 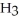 OH must be added to water to prepare 325 mL of a solution that is 4.50 M CH3OH?
OH must be added to water to prepare 325 mL of a solution that is 4.50 M CH3OH?
(Multiple Choice)
4.9/5  (39)
(39)
What is the concentration of NO3- ions in a solution prepared by dissolving 30.0 g of Ca(NO3)2 in enough water to produce 300.mL of solution?
(Multiple Choice)
4.8/5  (33)
(33)
Give the spectator ions for the reaction that occurs when aqueous solutions of H2SO4 and KOH are mixed.
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the number of grams of solute in 500.0 mL of 0.169 M KOH.
(Multiple Choice)
4.9/5  (46)
(46)
Determine the molarity of a solution formed by dissolving 4.00 moles of KBr in enough water to yield 3.00 L of solution.
(Multiple Choice)
4.9/5  (40)
(40)
Consider the following reaction.How many moles of oxygen are required to produce 2.00 moles of carbon dioxide? Assume that there is excess C3H7SH present. C3H7SH(l)+ 6 O2(g)→ 3 CO2(g)+ SO2(g)+ 4 H2O(g)
(Multiple Choice)
4.8/5  (35)
(35)
What element is undergoing reduction (if any)in the following reaction? Zn(s)+ 2 AgNO3(aq)→ Zn(NO3)2(aq)+ 2 Ag(s)
(Multiple Choice)
4.8/5  (34)
(34)
What is the concentration (M)of CH3OH in a solution prepared by dissolving 12.5 g of CH3OH in sufficient water to give exactly 230 mL of solution?
(Multiple Choice)
4.8/5  (40)
(40)
How many milliliters of 0.200 M FeCl3 are needed to react with an excess of Na2S to produce 0.690 g of Fe2S3 if the percent yield for the reaction is 65.0%? 3 Na2S(aq)+ 2 FeCl3(aq)→ Fe2S3(s)+ 6 NaCl(aq)
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following pairs of aqueous solutions will form a precipitate when mixed?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 181 - 200 of 239
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)