Exam 4: Chemical Quantities and Aqueous Reactions
Exam 1: Matter, measurement, and Problem Solving170 Questions
Exam 2: Atoms and Elements157 Questions
Exam 3: Molecules,compounds,and Chemical Equations175 Questions
Exam 4: Chemical Quantities and Aqueous Reactions239 Questions
Exam 5: Gases182 Questions
Exam 6: Thermochemistry143 Questions
Exam 7: The Quantum-Mechanical Model of the Atom134 Questions
Exam 8: Periodic Properties of the Elements147 Questions
Exam 9: Chemical Bonding I: Lewis Theory166 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,144 Questions
Exam 11: Liquids,solids,and Intermolecular Forces128 Questions
Exam 12: Solids and Modern Materials81 Questions
Exam 13: Solutions157 Questions
Exam 14: Chemical Kinetics154 Questions
Exam 15: Chemical Equilibrium141 Questions
Exam 16: Acids and Bases160 Questions
Exam 17: Aqueous Ionic Equilibrium187 Questions
Exam 18: Free Energy and Thermodynamics130 Questions
Exam 19: Electrochemistry151 Questions
Exam 20: Radioactivity and Nuclear Chemistry135 Questions
Exam 21: Organic Chemistry104 Questions
Exam 22: Biochemistry68 Questions
Exam 23: Chemistry of the Nonmetals66 Questions
Exam 24: Metals and Metallurgy60 Questions
Exam 25: Transition Metals and Coordination Compounds73 Questions
Select questions type
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6 Li(s)+ 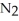 (g)→ 2 Li3N(s)
How many moles of lithium are needed to produce 0.31 mol of
(g)→ 2 Li3N(s)
How many moles of lithium are needed to produce 0.31 mol of 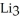 N when the reaction is carried out in the presence of excess nitrogen?
N when the reaction is carried out in the presence of excess nitrogen?
(Multiple Choice)
4.9/5  (40)
(40)
How many milliliters of 0.260 M Na2S are needed to react with 35.00 mL of 0.315 M AgNO3? Na2S(aq)+ 2 AgNO3(aq)→ 2 NaNO3(aq)+ Ag2S(s)
(Multiple Choice)
4.8/5  (31)
(31)
How many grams of H2 gas can be produced by the reaction of 63.0 grams of Al(s)with an excess of dilute hydrochloric acid in the reaction shown below? 2 Al(s)+ 6 HCl(aq)→ 2 AlCl3(aq)+ 3 H2(g)
(Multiple Choice)
4.9/5  (39)
(39)
If the percent yield for the following reaction is 75.0%,and 35.0 g of NO2 are consumed in the reaction,how many grams of nitric acid,HNO3(aq)are produced? 3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
(Multiple Choice)
4.9/5  (39)
(39)
What is the concentration of FeCl3 in a solution prepared by dissolving 10.0 g of FeCl3 in enough water to make 275 mL of solution?
(Multiple Choice)
4.9/5  (33)
(33)
How many milliliters of a 0.266 M KNO3 solution are required to make 150.0 mL of 0.075 M KNO3 solution?
(Multiple Choice)
4.7/5  (35)
(35)
How many grams of AgNO3 are needed to make 250.mL of a solution that is 0.140 M?
(Multiple Choice)
4.8/5  (35)
(35)
Consider the following balanced reaction.What mass (in g)of CO2 can be formed from 288 mg of O2? Assume that there is excess C3H7SH present. C3H7SH(l)+ 6 O2(g)→ 3 CO2(g)+ SO2(g)+ 4 H2O(g)
(Multiple Choice)
4.9/5  (36)
(36)
How many milliliters of 0.111 M HClO4 solution are needed to neutralize 50.00 mL of 0.0789 M NaOH?
(Multiple Choice)
4.7/5  (41)
(41)
How many moles of CH3CH2OH are contained in 548 mL of 0.0788 M CH3CH2OH solution?
(Multiple Choice)
4.8/5  (31)
(31)
What volume of 0.305 M AgNO3 is required to react exactly with 155.0 mL of 0.274 M Na2SO4 solution? Hint: You will want to write a balanced reaction.
(Multiple Choice)
4.8/5  (33)
(33)
What are the coefficients in front of NO3-(aq)and Ni(s)when the following redox equation is balanced in an acidic solution? ____ NO3-(aq)+ ____ Ni(s)→ ____ NO(g)+ ____ Ni2+(aq)
(Multiple Choice)
4.8/5  (43)
(43)
According to the balanced equation shown below,3.00 moles of oxalic acid,H2C2O4,react with ________ moles of permanganate,MnO4-. 5 H2C2O4(aq)+ 2 MnO4-(aq)+ 6 H+aq)→ 10 CO2(g)+ Mn2+(aq)+ 8 H2O(l)
(Multiple Choice)
4.7/5  (26)
(26)
When 15.6 mL of 0.500 M AgNO3 is added to 12.5 mL of 0.300 M NH4Cl,how many grams of AgCl are formed? AgNO3(aq)+ NH4Cl(aq)→ AgCl(s)+ NH4NO3(aq)
(Multiple Choice)
4.8/5  (34)
(34)
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s)+ H2O(l)→ Ca(OH)2(s)
A 1)00-g sample of CaO is reacted with 0.965 g of H2O.How many grams of water remain after the reaction is complete?
(Multiple Choice)
4.9/5  (48)
(48)
Showing 121 - 140 of 239
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)