Exam 4: Chemical Quantities and Aqueous Reactions
Exam 1: Matter, measurement, and Problem Solving170 Questions
Exam 2: Atoms and Elements157 Questions
Exam 3: Molecules,compounds,and Chemical Equations175 Questions
Exam 4: Chemical Quantities and Aqueous Reactions239 Questions
Exam 5: Gases182 Questions
Exam 6: Thermochemistry143 Questions
Exam 7: The Quantum-Mechanical Model of the Atom134 Questions
Exam 8: Periodic Properties of the Elements147 Questions
Exam 9: Chemical Bonding I: Lewis Theory166 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,144 Questions
Exam 11: Liquids,solids,and Intermolecular Forces128 Questions
Exam 12: Solids and Modern Materials81 Questions
Exam 13: Solutions157 Questions
Exam 14: Chemical Kinetics154 Questions
Exam 15: Chemical Equilibrium141 Questions
Exam 16: Acids and Bases160 Questions
Exam 17: Aqueous Ionic Equilibrium187 Questions
Exam 18: Free Energy and Thermodynamics130 Questions
Exam 19: Electrochemistry151 Questions
Exam 20: Radioactivity and Nuclear Chemistry135 Questions
Exam 21: Organic Chemistry104 Questions
Exam 22: Biochemistry68 Questions
Exam 23: Chemistry of the Nonmetals66 Questions
Exam 24: Metals and Metallurgy60 Questions
Exam 25: Transition Metals and Coordination Compounds73 Questions
Select questions type
8.0 g of iron is reacted with 8.0 g of water according to the chemical equation shown below.Which one of the following statements is FALSE? 3 Fe(s)+ 4 H2O(l)→ Fe3O4(s)+ 4 H2(g)
(Multiple Choice)
4.8/5  (27)
(27)
Carbonic acid can form water and carbon dioxide upon heating.How much carbon dioxide is formed from 1.55 g of carbonic acid? H2CO3 → H2O + CO2
(Multiple Choice)
4.8/5  (43)
(43)
What is the molarity of a potassium triiodide solution,KI3(aq),if 30.00 mL of the solution is required to completely react with 25.00 mL of a 0.500 M thiosulfate solution,K2S2O3(aq)? The chemical equation for the reaction is 2 S2O32-(aq)+ I3-(aq)→ S4O62-(aq)+ 3 I-(aq).
(Multiple Choice)
4.9/5  (30)
(30)
Determine the molarity of a solution formed by dissolving 468 mg of MgI2 in enough water to yield 50.0 mL of solution.
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following solutions will have the highest electrical conductivity?
(Multiple Choice)
5.0/5  (39)
(39)
If 100.mL of 0.100 M Na2SO4 is added to 200.mL of 0.150 M NaCl,what is the concentration of Na+ ions in the final solution? Assume that the volumes are additive.
(Multiple Choice)
4.8/5  (35)
(35)
How many milliliters of 0.550 M hydriodic acid are needed to react with 25.00 mL of 0.217 M CsOH?
HI(aq)+ CsOH(aq)→ CsI(aq)+ H2O(l)
(Multiple Choice)
4.8/5  (42)
(42)
Lithium and nitrogen react to produce lithium nitride: 6 Li(s)+ 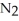 (g)→ 2 Li3N(s)
How many moles of
(g)→ 2 Li3N(s)
How many moles of 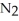 are needed to react with 0.550 mol of lithium?
are needed to react with 0.550 mol of lithium?
(Multiple Choice)
5.0/5  (28)
(28)
Determine the reducing agent in the following reaction. 2 Li(s)+ Fe(C2H3O2)2(aq)→ 2 LiC2H3O2(aq)+ Fe(s)
(Multiple Choice)
4.7/5  (25)
(25)
What is the concentration (M)of sodium ions in 4.57 L of a 2.35 M Li3P solution?
(Multiple Choice)
4.9/5  (32)
(32)
There are ________ mol of bromide ions in 0.300 L of a 0.150M solution of AlBr3.
(Multiple Choice)
4.9/5  (35)
(35)
Give the net ionic equation for the reaction (if any)that occurs when aqueous solutions of H2SO4 and KOH are mixed.
(Multiple Choice)
4.9/5  (41)
(41)
Give the net ionic equation for the reaction (if any)that occurs when aqueous solutions of Al(C2H3O2)3 and LiNO3 are mixed.
(Multiple Choice)
4.8/5  (50)
(50)
What volume (in mL)of 0.0887 M MgCl2 solution is needed to make 275.0 mL of 0.0224 M MgCl2 solution?
(Multiple Choice)
4.7/5  (37)
(37)
What is the concentration of an AlCl3 solution if 150.mL of the solution contains 250.mg of Cl- ion?
(Multiple Choice)
4.7/5  (44)
(44)
How many milliliters of a 0.184 M KNO3 solution contain 0.113 moles of KNO3?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 161 - 180 of 239
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)