Exam 7: The Quantum-Mechanical Model of the Atom
Exam 1: Matter, measurement, and Problem Solving170 Questions
Exam 2: Atoms and Elements157 Questions
Exam 3: Molecules,compounds,and Chemical Equations175 Questions
Exam 4: Chemical Quantities and Aqueous Reactions239 Questions
Exam 5: Gases182 Questions
Exam 6: Thermochemistry143 Questions
Exam 7: The Quantum-Mechanical Model of the Atom134 Questions
Exam 8: Periodic Properties of the Elements147 Questions
Exam 9: Chemical Bonding I: Lewis Theory166 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,144 Questions
Exam 11: Liquids,solids,and Intermolecular Forces128 Questions
Exam 12: Solids and Modern Materials81 Questions
Exam 13: Solutions157 Questions
Exam 14: Chemical Kinetics154 Questions
Exam 15: Chemical Equilibrium141 Questions
Exam 16: Acids and Bases160 Questions
Exam 17: Aqueous Ionic Equilibrium187 Questions
Exam 18: Free Energy and Thermodynamics130 Questions
Exam 19: Electrochemistry151 Questions
Exam 20: Radioactivity and Nuclear Chemistry135 Questions
Exam 21: Organic Chemistry104 Questions
Exam 22: Biochemistry68 Questions
Exam 23: Chemistry of the Nonmetals66 Questions
Exam 24: Metals and Metallurgy60 Questions
Exam 25: Transition Metals and Coordination Compounds73 Questions
Select questions type
Calculate the energy of the orange light emitted,per photon,by a neon sign with a frequency of 4.89 × 1014 Hz.
(Multiple Choice)
4.7/5  (36)
(36)
Each of the following sets of quantum numbers is supposed to specify an orbital.Choose the one set of quantum numbers that does NOT contain an error.
(Multiple Choice)
4.9/5  (41)
(41)
Determine the energy change associated with the transition from 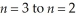 in the hydrogen atom.
in the hydrogen atom.
(Multiple Choice)
4.8/5  (40)
(40)
Determine the energy change associated with the transition from 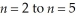 in the hydrogen atom.
in the hydrogen atom.
(Multiple Choice)
5.0/5  (46)
(46)
Determine the end (final)value of n in a hydrogen atom transition,if the electron starts in n = 1 and the atom absorbs a photon of light with an energy of 2.044 × 10-18 J.
(Multiple Choice)
4.8/5  (34)
(34)
When an electric current is passed through a tube containing neon,neon atoms absorb some of the electrical energy and reemit it as a ________ color.
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following visible colors of light has the longest wavelength?
(Multiple Choice)
4.7/5  (40)
(40)
Determine the velocity of a medicine ball (m = 10.0 kg)with a wavelength of 1.33 × 10-35 m.
(Multiple Choice)
5.0/5  (28)
(28)
Determine the shortest frequency of light required to remove an electron from a sample of Ti metal if the binding energy of titanium is 3.14 × 103 kJ/mol.
(Multiple Choice)
4.7/5  (36)
(36)
The number of cycles that pass through a stationary point is called
(Multiple Choice)
4.9/5  (42)
(42)
Place the following types of electromagnetic radiation in order of decreasing energy. visible light radio waves infrared light
(Multiple Choice)
5.0/5  (31)
(31)
How many photons are contained in a burst of yellow light (589 nm)from a sodium lamp that contains 623 kJ of energy?
(Multiple Choice)
4.8/5  (44)
(44)
During a thunderstorm,you see a flash of lightning.Five seconds later,you hear the thunder.How far away is the storm?
(Multiple Choice)
4.7/5  (32)
(32)
Calculate the energy of the green light emitted,per photon,by a mercury lamp with a frequency of 5.49 × 1014 Hz.
(Multiple Choice)
4.9/5  (36)
(36)
How much energy (in kJ)is required to ionize 2.52 moles of hydrogen atoms?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 41 - 60 of 134
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)