Exam 18: Nucleotide Metabolism
Exam 1: Principles of Biochemistry98 Questions
Exam 2: Physical Biochemistry: Energy Conversion,water,and Membranes99 Questions
Exam 3: Nucleic Acid Structure and Function100 Questions
Exam 4: Protein Structure100 Questions
Exam 5: Methods in Protein Biochemistry98 Questions
Exam 6: Protein Function113 Questions
Exam 7: Enzyme Mechanisms105 Questions
Exam 8: Cell Signaling Systems102 Questions
Exam 9: Glycolysis: a Paradigm of Metabolic Regulation100 Questions
Exam 10: The Citrate Cycle100 Questions
Exam 11: Oxidative Phosphorylation98 Questions
Exam 12: Photosynthesis100 Questions
Exam 13: Carbohydrate Structure and Function100 Questions
Exam 14: Carbohydrate Metabolism100 Questions
Exam 15: Lipid Structure and Function98 Questions
Exam 16: Lipid Metabolism100 Questions
Exam 17: Amino Acid Metabolism100 Questions
Exam 18: Nucleotide Metabolism98 Questions
Exam 19: Metabolic Integration101 Questions
Exam 20: Dna Replication, repair, and Recombination99 Questions
Exam 21: Rna Synthesis, processing, and Gene Silencing100 Questions
Exam 22: Protein Synthesis, posttranslational Modification, and Transport100 Questions
Exam 23: Gene Regulation99 Questions
Select questions type
Tyr122 is important in the mechanism of the E.coli ribonucleotide reductase.The function of this amino acid in the mechanism is to
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
C
During the biosynthesis of UMP from carbamoyl phosphate and aspartate,dihydroorotate is converted to which of the following compounds?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
B
Contrast the source of the nitrogen required for the conversion of UTP to CTP in humans and bacteria.
Free
(Essay)
4.7/5  (35)
(35)
Correct Answer:
Humans use glutamine,whereas bacteria use ammonia (NH4+).
The activity site of ribonucleotide reductase can bind two different molecules,whereas the specificity site can bind several.Construct a situation where the activity of the enzyme is inhibited by choosing the identity of the molecules bound to each site.
(Essay)
4.8/5  (34)
(34)
Two biosynthesis steps are necessary to incorporate the carbon from HCO3 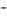 into carbamoyl aspartate.Describe the intermediate compound generated during this conversion.
into carbamoyl aspartate.Describe the intermediate compound generated during this conversion.
(Essay)
4.9/5  (36)
(36)
Consider the breakdown of the four compounds below.Which would be expected to generate 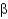 -alanine?
-alanine?
(Multiple Choice)
4.9/5  (40)
(40)
To determine the role of glutaredoxin in the generation of deoxyribonucleotides by ribonucleotide reductase,which organisms would be the best to study?
(Multiple Choice)
4.8/5  (35)
(35)
Several cysteines play important roles in the mechanism of E.coli ribonucleotide reductase.Which is NOT a role carried out by cysteine in this enzyme?
(Multiple Choice)
4.9/5  (37)
(37)
Analysis of enzyme activities in a cell in the absence and presence of AMP and GMP is completed.Which enzyme would show lower activity when AMP is added but not when GMP is added?
(Multiple Choice)
5.0/5  (37)
(37)
Glutamine-PRPP amidotransferase is a tetrameric enzyme that displays sigmoidal kinetics when no inhibitors are present.In the presence of either GTP or ATP the kinetics curve is sigmoidal.Why does this difference in kinetics occur?
(Short Answer)
4.8/5  (34)
(34)
Analysis of AMP and GMP synthesized in E.coli grown in medium containing 15N-labeled aspartate would show that
(Multiple Choice)
4.8/5  (36)
(36)
The transformation of ribose-5-phosphate to AIR in the first stage of purine biosynthesis in E.coli consumes the equivalent of 5 ATP.The second stage consumes
(Multiple Choice)
4.8/5  (35)
(35)
The nitrogen atoms of a purine originate from all of the following amino acids EXCEPT
(Multiple Choice)
4.9/5  (31)
(31)
Consider a cell that is provided aspartate in which all carbons are radioactively labeled.Compounds in the cell are analyzed after the biosynthesis and subsequent breakdown of pyrimidines.Which of the following compounds would display no radioactivity signal during the analysis?
(Multiple Choice)
4.9/5  (34)
(34)
Explain how the feedback inhibition of IMP dehydrogenase by GMP can increase the flux of the ATP synthesis pathway.
(Essay)
4.9/5  (36)
(36)
When analyzing the production of dTMP by thymidylate synthase in a cell-free system,which of the following compounds would be found as an additional product of this enzyme?
(Multiple Choice)
4.8/5  (41)
(41)
What would be the effect on thymidylate synthase activity if raltitrexed is present?
(Essay)
4.8/5  (43)
(43)
Which answer INCORRECTLY pairs a substrate and subsequent product of dihydropyrimidine dehydrogenase?
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following is a coenzyme that is derived from ATP?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)