Exam 1: Principles of Biochemistry
Exam 1: Principles of Biochemistry98 Questions
Exam 2: Physical Biochemistry: Energy Conversion,water,and Membranes99 Questions
Exam 3: Nucleic Acid Structure and Function100 Questions
Exam 4: Protein Structure100 Questions
Exam 5: Methods in Protein Biochemistry98 Questions
Exam 6: Protein Function113 Questions
Exam 7: Enzyme Mechanisms105 Questions
Exam 8: Cell Signaling Systems102 Questions
Exam 9: Glycolysis: a Paradigm of Metabolic Regulation100 Questions
Exam 10: The Citrate Cycle100 Questions
Exam 11: Oxidative Phosphorylation98 Questions
Exam 12: Photosynthesis100 Questions
Exam 13: Carbohydrate Structure and Function100 Questions
Exam 14: Carbohydrate Metabolism100 Questions
Exam 15: Lipid Structure and Function98 Questions
Exam 16: Lipid Metabolism100 Questions
Exam 17: Amino Acid Metabolism100 Questions
Exam 18: Nucleotide Metabolism98 Questions
Exam 19: Metabolic Integration101 Questions
Exam 20: Dna Replication, repair, and Recombination99 Questions
Exam 21: Rna Synthesis, processing, and Gene Silencing100 Questions
Exam 22: Protein Synthesis, posttranslational Modification, and Transport100 Questions
Exam 23: Gene Regulation99 Questions
Select questions type
Describe the differences between the structures of pyrimidine and a purine.
Free
(Essay)
4.8/5  (27)
(27)
Correct Answer:
A pyrimidine is an aromatic molecule with nitrogen at positions 1 and 3 on the ring,along with a carbonyl at position 4.Examples of pyrimidines are cytosine,thymine,and uracil.A purine is a heterocyclic aromatic molecule with nitrogen at positions 1,3,7,and 9.Examples of purines are guanine and adenine.
Hydrogen bonds form between hydrogen and
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
A
ATP is an abbreviation for which energy currency molecule?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
A
Look at the bond energies O-H,N-H,and P-H in the table below.O-H is the hardest bond to break because it has the 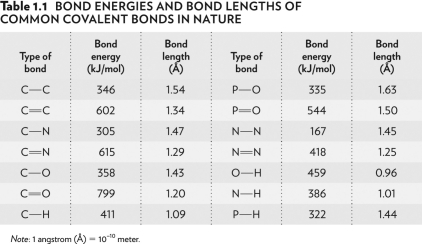
(Multiple Choice)
4.9/5  (44)
(44)
The "central dogma of molecular biology" can best be described as the transfer of information between
(Multiple Choice)
4.9/5  (33)
(33)
The figure below shows an example of which functional group? 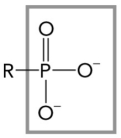
(Multiple Choice)
4.9/5  (39)
(39)
If a mutation was made to the gene for glucose-6-phosphate dehydrogenase that prevented it from functioning,a possible outcome would be the production of
(Multiple Choice)
4.8/5  (39)
(39)
Amino acids are the building blocks for which biomolecule(s)?
(Multiple Choice)
4.9/5  (41)
(41)
Who received the Nobel Prize in 1962 for elucidating the molecular structure of DNA?
(Multiple Choice)
4.8/5  (34)
(34)
The correct name for the VSEPR arrangement around a carbon in methane is
(Multiple Choice)
4.9/5  (38)
(38)
Give an example of each of the following: element,biomolecule,macromolecule,metabolism,cell,organism,and ecosystem.
(Essay)
4.9/5  (37)
(37)
Describe how a receptor is activated and inactivated by a ligand in the figure below. 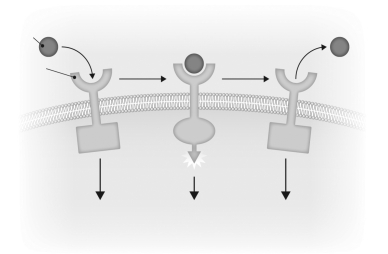
(Essay)
4.8/5  (37)
(37)
The DNA double helix is stabilized by the interactions between nucleotides because of __________ between nucleotides.
(Multiple Choice)
4.8/5  (38)
(38)
Amino acids are the building blocks of proteins.There are 20 amino acids; how do these amino acids differ from one another?
(Essay)
4.8/5  (37)
(37)
Showing 1 - 20 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)