Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides
Exam 1: Carbon Compounds and Chemical Bonds134 Questions
Exam 2: Representative Carbon Compounds: Functional Groups, Intermolecular Forces, and Infrared Ir Spectroscopy114 Questions
Exam 3: An Introduction to Organic Reactions: Acids and Bases47 Questions
Exam 4: Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis125 Questions
Exam 5: Stereochemistry: Chiral Molecules150 Questions
Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides146 Questions
Exam 7: Alkenes and Alkynes I: Properties and Synthesis, Elimination Reactions of Alkyl Halides99 Questions
Exam 8: Alkenes and Alkynes Ii: Addition Reactions140 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination94 Questions
Exam 10: Radical Reactions114 Questions
Exam 11: Alcohols and Ethers172 Questions
Exam 12: Alcohols From Carbonyl Compounds Oxidation-Reduction and Organometallic Compounds147 Questions
Exam 13: Conjugated Unsaturated Systems166 Questions
Exam 14: Aromatic Compounds151 Questions
Exam 15: Reactions of Aromatic Compounds173 Questions
Exam 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group165 Questions
Exam 17: Aldehydes and Ketones Ii Aldol Reactions131 Questions
Exam 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon124 Questions
Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions131 Questions
Exam 20: Amines148 Questions
Exam 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution87 Questions
Exam 22: Carbohydrates104 Questions
Exam 23: Lipids99 Questions
Exam 24: Amino Acids and Proteins94 Questions
Exam 25: Nucleic Acids and Protein Synthesis89 Questions
Select questions type
What would be the major product(s)of the following reaction? 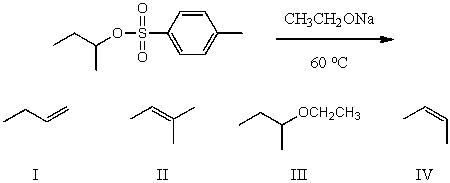
(Multiple Choice)
4.9/5  (41)
(41)
Draw the potential energy diagram that represents an exothermic reaction between a tertiary alkyl halide and methanol.Briefly explain your rationale.
(Essay)
4.9/5  (41)
(41)
Consider the reaction of 2-chloro-2-methylpentane with sodium iodide. 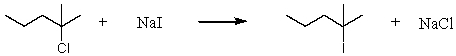 Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
(Multiple Choice)
4.8/5  (31)
(31)
By analyzing the starting material and the product(s),the following reaction is possibly an example of what type of mechanism? 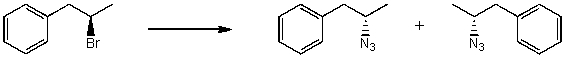
(Multiple Choice)
4.8/5  (40)
(40)
The substitution mechanism whose rate depends primarily on the degree of steric hindrance around the leaving group is the _________.
(Essay)
4.7/5  (33)
(33)
Which is the weakest nucleophile in polar aprotic solvents?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following would be most reactive in an SN2 reaction?
(Multiple Choice)
4.7/5  (40)
(40)
What would be the major product(s)of the following reaction? 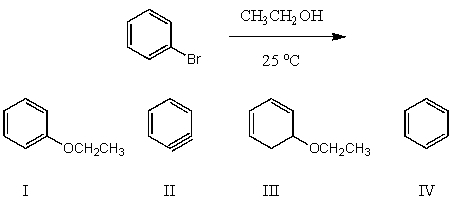
(Multiple Choice)
4.9/5  (33)
(33)
Select the potential energy diagram that represents an exothermic (exergonic)reaction. 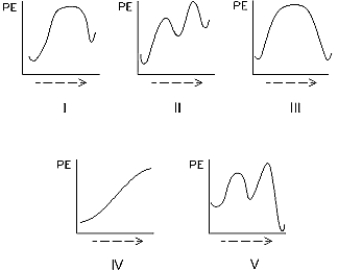
(Multiple Choice)
4.8/5  (35)
(35)
What major product(s)are likely to be obtained from the following reaction? 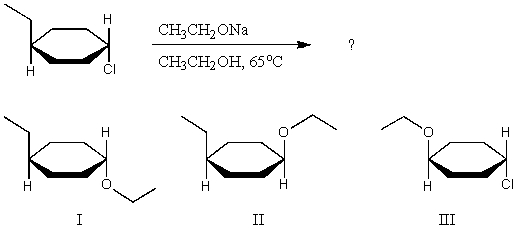
(Multiple Choice)
4.8/5  (34)
(34)
Which alkyl halide,when treated with sodium ethoxide in ethanol,would afford a product mixture consisting of more than one elimination product?
(Multiple Choice)
4.8/5  (40)
(40)
By analyzing the starting material and the product,the following reaction is possibly an example of what type of mechanism(s)? 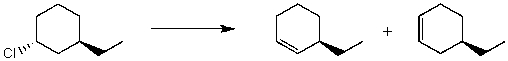
(Multiple Choice)
4.8/5  (41)
(41)
What would be the major product(s)of the following reaction? 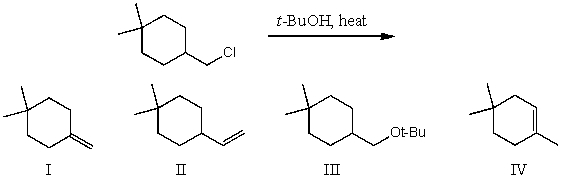
(Multiple Choice)
4.8/5  (30)
(30)
Which of these species,acting in a protic solvent,exhibits greater nucleophilic activity than expected on the basis of its basicity?
(Multiple Choice)
4.9/5  (43)
(43)
What would be the major product(s)of the following reaction? 
(Multiple Choice)
4.9/5  (44)
(44)
The relative nucleophilicity of the halide ions in polar aprotic solvents is observed to be markedly different from that in protic solvents.Explain briefly.
(Essay)
4.9/5  (31)
(31)
Which alkyl chloride,though primary,is essentially unreactive in SN2 reactions?
(Multiple Choice)
5.0/5  (34)
(34)
Reaction of sodium ethoxide with 1-bromopentane at 30 C yields primarily:
(Multiple Choice)
4.8/5  (34)
(34)
Heating an alcoholic solution of sodium hydroxide and 1-bromopentane at 60 C yields primarily:
(Multiple Choice)
4.9/5  (35)
(35)
Showing 101 - 120 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)