Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination
Exam 1: Carbon Compounds and Chemical Bonds134 Questions
Exam 2: Representative Carbon Compounds: Functional Groups, Intermolecular Forces, and Infrared Ir Spectroscopy114 Questions
Exam 3: An Introduction to Organic Reactions: Acids and Bases47 Questions
Exam 4: Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis125 Questions
Exam 5: Stereochemistry: Chiral Molecules150 Questions
Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides146 Questions
Exam 7: Alkenes and Alkynes I: Properties and Synthesis, Elimination Reactions of Alkyl Halides99 Questions
Exam 8: Alkenes and Alkynes Ii: Addition Reactions140 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination94 Questions
Exam 10: Radical Reactions114 Questions
Exam 11: Alcohols and Ethers172 Questions
Exam 12: Alcohols From Carbonyl Compounds Oxidation-Reduction and Organometallic Compounds147 Questions
Exam 13: Conjugated Unsaturated Systems166 Questions
Exam 14: Aromatic Compounds151 Questions
Exam 15: Reactions of Aromatic Compounds173 Questions
Exam 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group165 Questions
Exam 17: Aldehydes and Ketones Ii Aldol Reactions131 Questions
Exam 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon124 Questions
Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions131 Questions
Exam 20: Amines148 Questions
Exam 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution87 Questions
Exam 22: Carbohydrates104 Questions
Exam 23: Lipids99 Questions
Exam 24: Amino Acids and Proteins94 Questions
Exam 25: Nucleic Acids and Protein Synthesis89 Questions
Select questions type
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 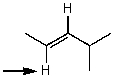
(Multiple Choice)
4.8/5  (37)
(37)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C5H11NO? In the 13C-NMR spectrum you notice that the farthest peak downfield has a chemical shift of 173 ppm.Relative integration is shown. 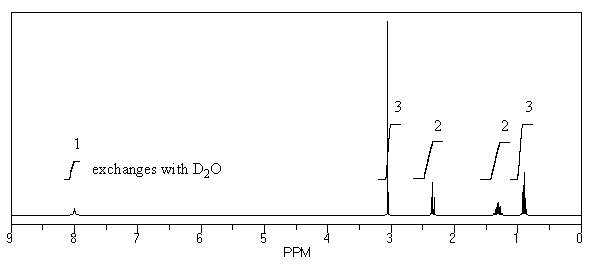
(Multiple Choice)
4.8/5  (37)
(37)
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 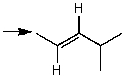
(Multiple Choice)
4.8/5  (28)
(28)
A mass spectrometer sorts ions on the basis of their _______________.
(Short Answer)
4.8/5  (41)
(41)
In electron impact mass spectrometry,a beam of high-energy electrons initially dislodges one electron from the compound being studied.This produces a positively charged ion called the ____________________.
(Short Answer)
4.8/5  (30)
(30)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C8H14? Relative integration is shown. 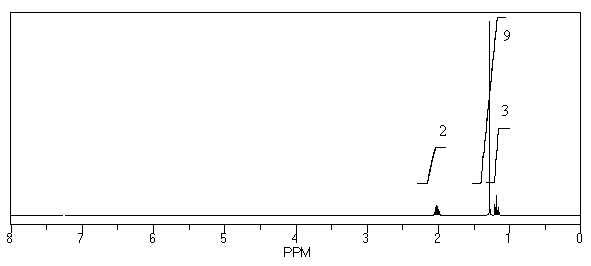
(Multiple Choice)
4.8/5  (41)
(41)
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 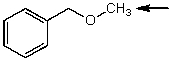
(Multiple Choice)
4.8/5  (37)
(37)
A compound with the molecular formula C8H9ClO gave the following 1H NMR spectrum: triplet, 3.7
Triplet, 4.2
Multiplet 7.1
There was no evidence of an -OH band in the IR spectrum.The most likely structure for the compound is: 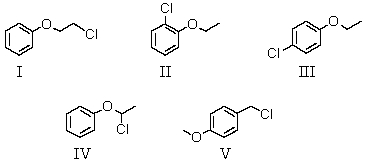
(Multiple Choice)
4.9/5  (35)
(35)
A compound with the molecular formula C8H9BrO gave the following 1H NMR spectrum: triplet, 1.4
Quartet, 3.9
Multiplet, 7.0 (4H)
There was no evidence of an -OH band in the IR spectrum.A possible structure for the compound is: 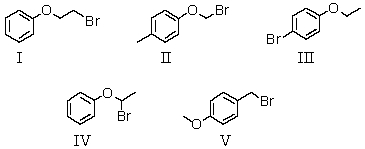
(Multiple Choice)
4.9/5  (36)
(36)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C10H11N? Looking at the 13C-NMR you notice 10 distinct peaks,and the IR has a characteristic peak around 2250 cm-1.Relative integration is shown. 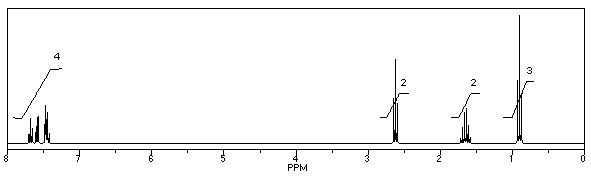
(Multiple Choice)
4.9/5  (38)
(38)
The 1H NMR spectrum of which of these compounds would consist of a triplet,singlet and quartet only?
(Multiple Choice)
4.8/5  (33)
(33)
Predict the splitting pattern you would observe for the proton at C3 of 2,3-dimethyl-2-phenylbutane. 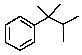
(Multiple Choice)
4.9/5  (43)
(43)
A compound C5H10O gave the following spectral data: 1H NMR spectrum IR spectrum
Doublet, 1.10 strong peak
Singlet, 2.10 near 1720 cm-1
Septet, 2.50
Which is a reasonable structure for the compound? 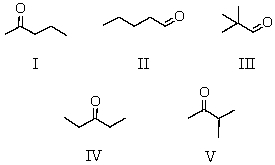
(Multiple Choice)
4.7/5  (33)
(33)
A compound with the molecular formula C10H13Cl gave the following 1H NMR spectrum: singlet, 1.6
Singlet, 3.1
Multiplet, 7.2 (5H)
The most likely structure for the compound is: 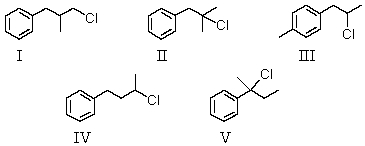
(Multiple Choice)
4.9/5  (35)
(35)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C10H12O2? Relative integration is shown. 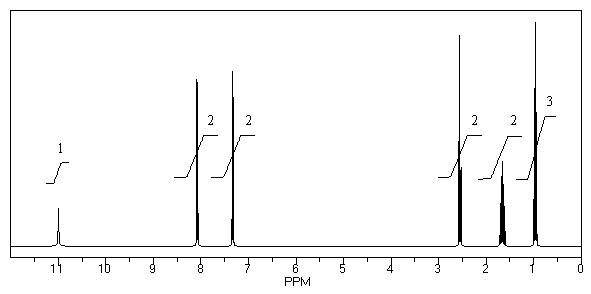
(Multiple Choice)
4.9/5  (28)
(28)
How many signals will be recorded in the broadband proton-decoupled 13C spectrum of 4-chloro-1-ethylbenzene? 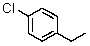
(Multiple Choice)
4.7/5  (36)
(36)
Consider the expected splitting of signal "b" in the 1H NMR spectrum of 1-methoxy-2-methylpropane,shown below.Presuming that Jab is sufficiently different from Jbc and that the instrument has sufficient resolving power,what is the theoretical multiplicity of signal "b"? 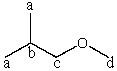
(Multiple Choice)
4.8/5  (38)
(38)
What can be determined from the relative abundance of the M + +1 peak?
(Essay)
4.8/5  (34)
(34)
Showing 61 - 80 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)