Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination
Exam 1: Carbon Compounds and Chemical Bonds134 Questions
Exam 2: Representative Carbon Compounds: Functional Groups, Intermolecular Forces, and Infrared Ir Spectroscopy114 Questions
Exam 3: An Introduction to Organic Reactions: Acids and Bases47 Questions
Exam 4: Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis125 Questions
Exam 5: Stereochemistry: Chiral Molecules150 Questions
Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides146 Questions
Exam 7: Alkenes and Alkynes I: Properties and Synthesis, Elimination Reactions of Alkyl Halides99 Questions
Exam 8: Alkenes and Alkynes Ii: Addition Reactions140 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination94 Questions
Exam 10: Radical Reactions114 Questions
Exam 11: Alcohols and Ethers172 Questions
Exam 12: Alcohols From Carbonyl Compounds Oxidation-Reduction and Organometallic Compounds147 Questions
Exam 13: Conjugated Unsaturated Systems166 Questions
Exam 14: Aromatic Compounds151 Questions
Exam 15: Reactions of Aromatic Compounds173 Questions
Exam 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group165 Questions
Exam 17: Aldehydes and Ketones Ii Aldol Reactions131 Questions
Exam 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon124 Questions
Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions131 Questions
Exam 20: Amines148 Questions
Exam 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution87 Questions
Exam 22: Carbohydrates104 Questions
Exam 23: Lipids99 Questions
Exam 24: Amino Acids and Proteins94 Questions
Exam 25: Nucleic Acids and Protein Synthesis89 Questions
Select questions type
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H12O which shows no characteristic stretches in the IR between 3600-3300 cm-1,but does around 1600 cm-1? Relative integration is shown. 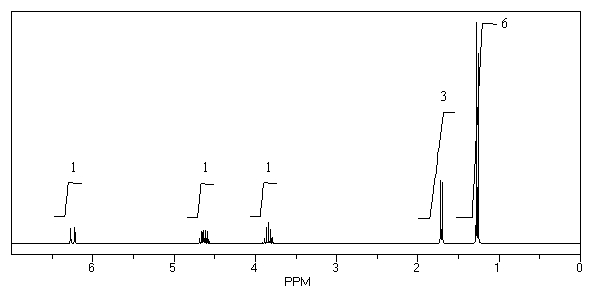
(Multiple Choice)
4.9/5  (34)
(34)
Which proton(s)of the compound below would appear as a septet in the 1H NMR spectrum? 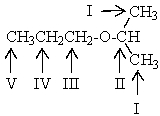
(Multiple Choice)
4.8/5  (36)
(36)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C11H14O2? Looking at the 13C-NMR you notice a peak at 174 ppm.Relative integration is shown. 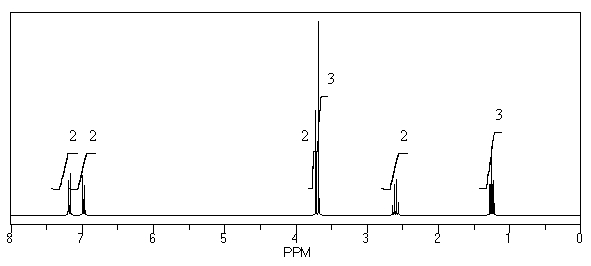
(Multiple Choice)
4.9/5  (46)
(46)
What is the molecular formula of this compound? m/z
Intensity
78 M +
10)00
79
1
80
3)3
81
0)3
(Multiple Choice)
4.7/5  (41)
(41)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C5H11NO,which shows a characteristic stretch in the IR around 1700 cm-1,and a characteristic peak at 202 ppm in the 13C-NMR? Relative integration is shown. 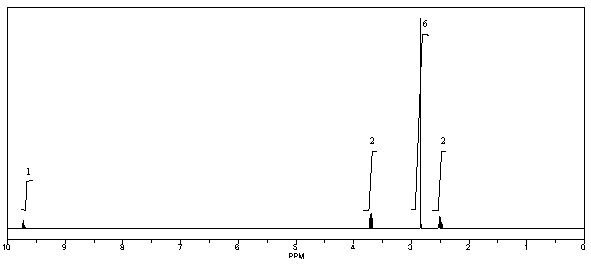
(Multiple Choice)
5.0/5  (38)
(38)
The broadband proton-decoupled 13C NMR spectrum of a hexyl chloride exhibits five signals.Which of these structures could be the correct one for the compound?
(Multiple Choice)
4.8/5  (38)
(38)
Which form of electromagnetic radiation possesses the least energy?
(Short Answer)
4.9/5  (38)
(38)
Which proton(s)of the compound below would appear as a triplet in the 1H NMR spectrum? 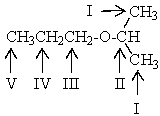
(Multiple Choice)
4.8/5  (35)
(35)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H10O2? The 13C-NMR shows characteristic chemical shifts at 22.3,31.1,117.3,157.7,and 171.6 ppm.Relative integration is shown. 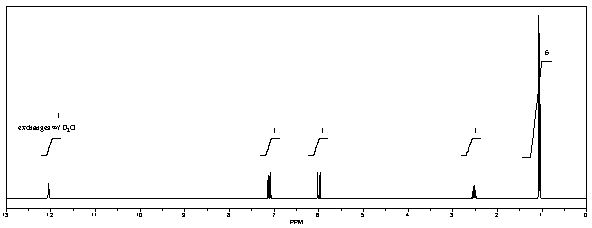
(Multiple Choice)
4.8/5  (40)
(40)
What compound is used as the standard "zero" reference in both carbon and proton NMR?
(Essay)
4.8/5  (43)
(43)
A shielded proton will absorb at a higher frequency (this is the ________ end of the spectrum);and a deshielded proton will absorb at a lower frequency (the _____________ end of the spectrum).
(Short Answer)
4.9/5  (31)
(31)
If all the protons of 1-fluoropentane could be discerned,which would you expect to be at the lowest field in the 1H NMR spectrum of this compound? 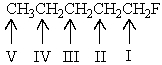
(Multiple Choice)
4.8/5  (30)
(30)
A downfield ( 9-10)singlet is observed in the 1H NMR spectrum of:
(Multiple Choice)
4.9/5  (31)
(31)
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C9H18O2 and characteristic 13C-NMR peaks at 11.3,21.6,25.3,49.4,67.1,and 175.5 ppm? Relative integration is shown. 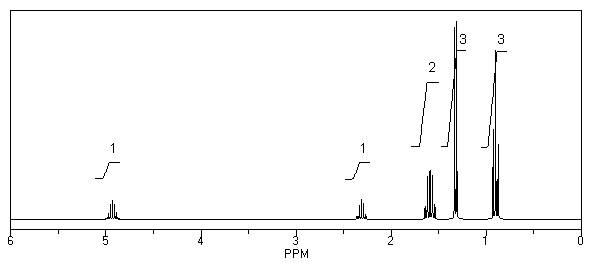
(Multiple Choice)
4.8/5  (31)
(31)
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 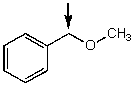
(Multiple Choice)
4.8/5  (32)
(32)
The 1H NMR spectrum of which of the compounds below,all of formula C7H12O2,would consist of two singlets only? 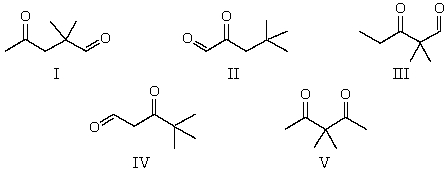
(Multiple Choice)
4.8/5  (37)
(37)
An organic compound absorbs strongly in the IR at 1687 cm-1.Its 1H NMR spectrum consists of two signals,a singlet at 2.1 ppm and a multiplet centered at 7.1 ppm.Its mass spectrum shows significant peaks at m/z 120,m/z 105 and m/z 77.This information is consistent with which of the following structures? 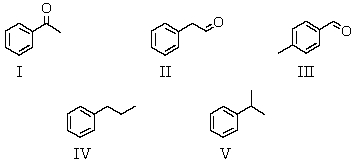
(Multiple Choice)
4.9/5  (36)
(36)
Which of these compounds will not be represented by a singlet only in the 1H NMR spectrum?
(Multiple Choice)
4.8/5  (22)
(22)
Showing 21 - 40 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)