Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions
Exam 1: Carbon Compounds and Chemical Bonds134 Questions
Exam 2: Representative Carbon Compounds: Functional Groups, Intermolecular Forces, and Infrared Ir Spectroscopy114 Questions
Exam 3: An Introduction to Organic Reactions: Acids and Bases47 Questions
Exam 4: Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis125 Questions
Exam 5: Stereochemistry: Chiral Molecules150 Questions
Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides146 Questions
Exam 7: Alkenes and Alkynes I: Properties and Synthesis, Elimination Reactions of Alkyl Halides99 Questions
Exam 8: Alkenes and Alkynes Ii: Addition Reactions140 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination94 Questions
Exam 10: Radical Reactions114 Questions
Exam 11: Alcohols and Ethers172 Questions
Exam 12: Alcohols From Carbonyl Compounds Oxidation-Reduction and Organometallic Compounds147 Questions
Exam 13: Conjugated Unsaturated Systems166 Questions
Exam 14: Aromatic Compounds151 Questions
Exam 15: Reactions of Aromatic Compounds173 Questions
Exam 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group165 Questions
Exam 17: Aldehydes and Ketones Ii Aldol Reactions131 Questions
Exam 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon124 Questions
Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions131 Questions
Exam 20: Amines148 Questions
Exam 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution87 Questions
Exam 22: Carbohydrates104 Questions
Exam 23: Lipids99 Questions
Exam 24: Amino Acids and Proteins94 Questions
Exam 25: Nucleic Acids and Protein Synthesis89 Questions
Select questions type
Which reagents would you use to synthesize this compound by an aldol condensation? 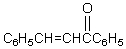
(Multiple Choice)
4.7/5  (35)
(35)
Esters frequently react in the presence of alkoxides by a reaction called the __________ condensation.
(Short Answer)
4.9/5  (33)
(33)
What final product is obtained when 2,8-nonanone is treated with base,followed by reaction with sodium borohydride? 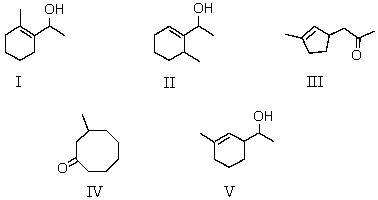
(Multiple Choice)
4.9/5  (34)
(34)
When , -unsaturated aldehydes and ketones react with nucleophiles,simple addition is favored when _________ nucleophiles are used,while conjugate addition is preferred by ___________ nucleophiles.
(Short Answer)
4.9/5  (38)
(38)
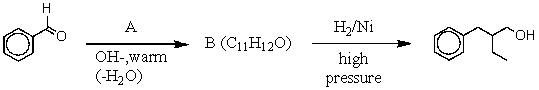 -Consider the synthesis above in answering this question.What is compound A?
-Consider the synthesis above in answering this question.What is compound A?
(Multiple Choice)
4.9/5  (39)
(39)
What is the final product of the following reaction sequence? Give structural details of all significant intermediates. 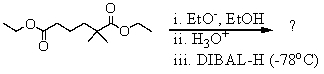
(Essay)
4.9/5  (40)
(40)
The aldol reaction of cyclohexanone produces which of these self-condensation products? 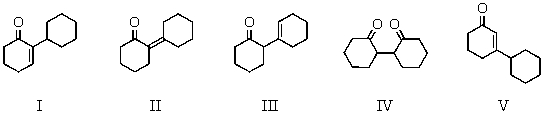
(Multiple Choice)
4.8/5  (38)
(38)
What is the final product of the following reaction sequence? Give structural
details of all significant intermediates. 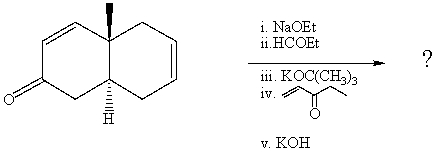
(Essay)
4.8/5  (37)
(37)
Which of the following would afford the best synthesis of diethyl phenylmalonate, 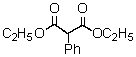
(Multiple Choice)
4.7/5  (39)
(39)
The sequence of a Michael addition followed by an intramolecular aldol condensation is known as a ___________.
(Essay)
4.9/5  (35)
(35)
Which reagents would be used in a Mannich reaction to synthesize 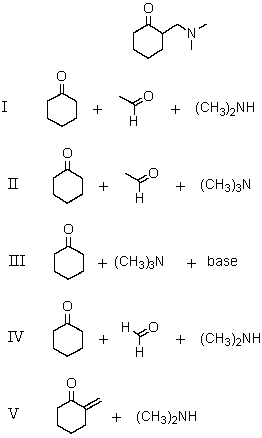
(Multiple Choice)
4.9/5  (36)
(36)
Which of these is a product of the reaction of C6H5MgBr with 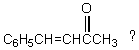
(Multiple Choice)
4.7/5  (40)
(40)
Which is the only one of these compounds which cannot self-condense in the presence of dilute aqueous alkali?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 80 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)