Exam 18: Heat and the First Law of Thermodynamics
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension83 Questions
Exam 3: Motion in Two and Three Dimensions60 Questions
Exam 4: Newtons Laws106 Questions
Exam 5: Applications of Newtons Laws73 Questions
Exam 6: Work and Energy60 Questions
Exam 7: Conservation of Energy56 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum92 Questions
Exam 9: Rotation105 Questions
Exam 10: Conservation of Angular Momentum66 Questions
Exam 11: Gravity84 Questions
Exam 12: Static Equilibrium and Elasticity58 Questions
Exam 13: Fluids77 Questions
Exam 14: Oscillations126 Questions
Exam 15: Wave Motion112 Questions
Exam 16: Superposition and Standing Waves87 Questions
Exam 17: Temperature and the Kinetic Theory of Gases78 Questions
Exam 18: Heat and the First Law of Thermodynamics100 Questions
Exam 19: The Second Law of Thermodynamics59 Questions
Exam 20: Thermal Properties and Processes50 Questions
Exam 21: The Electric Field I: Discrete Charge Distributions55 Questions
Exam 22: The Electric Field Ii: Continuous Charge Distributions64 Questions
Exam 23: Electric Potential87 Questions
Exam 24: Capacitance63 Questions
Exam 25: Electric Current and Direct-Current Circuits107 Questions
Exam 26: The Magnetic Field33 Questions
Exam 27: Sources of the Magnetic Field86 Questions
Exam 28: Magnetic Induction56 Questions
Exam 29: Alternating-Current Circuits106 Questions
Exam 30: Maxwells Equations and Electromagnetic Waves57 Questions
Exam 31: Properties of Light82 Questions
Exam 32: Optical Images106 Questions
Exam 33: Interference and Diffraction91 Questions
Exam 34: Wave Particle Duality and Quantum Physics140 Questions
Exam 35: Applications of the Schrodinger Equation42 Questions
Exam 36: Atoms113 Questions
Exam 37: Molecules39 Questions
Exam 38: Solids and the Theory of Conduction75 Questions
Exam 39: Relativity82 Questions
Exam 40: Nuclear Physics107 Questions
Exam 41: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
The first law of thermodynamics has as its basis the same fundamental principle as
(Multiple Choice)
4.7/5  (43)
(43)
Two liquids,A and B,are mixed together.Liquid A has mass m and was initially at temperature 40 C,and liquid B has mass 2m and was initially at temperature 5 C.The specific heat of liquid A is 1.5 times that of liquid B. Calculate the final temperature of the mixture.
(Multiple Choice)
4.8/5  (36)
(36)
A small lake has a surface area of 10000 m2.Assuming that the average depth of the lake is 2 m,how much heat is released when the average temperature of the water in the lake drops by 1C ?
(Multiple Choice)
4.9/5  (31)
(31)
An ideal gas with an initial volume of 3 L at a pressure of 2 atm is compressed adiabatically until it has a volume of 2 L; then it is cooled at constant volume until its temperature drops to its initial value.The final pressure is
(Multiple Choice)
5.0/5  (23)
(23)
The equation of state for a certain gas under isothermal conditions is  where the units are SI.The work done by this gas as its volume increases isothermally from 0.2 m3 to 0.8 m3 is approximately
where the units are SI.The work done by this gas as its volume increases isothermally from 0.2 m3 to 0.8 m3 is approximately
(Multiple Choice)
4.9/5  (38)
(38)
If the heat given off by 300 g of an alloy as it cools through 50 Cº is sufficient to raise the temperature of 300 g of water from 30º to 40ºC,the specific heat of the alloy must be approximately
(Multiple Choice)
4.9/5  (31)
(31)
Use the following to answer question: 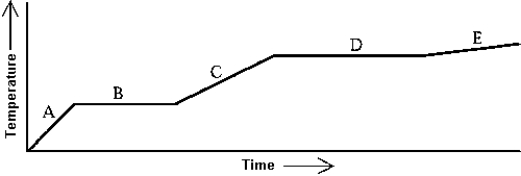 -Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The latent heat of vaporization can be found by
-Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The latent heat of vaporization can be found by
(Multiple Choice)
4.7/5  (28)
(28)
A lake with 8.0 109 kg of water,which has a specific heat of 4180 J/kg · Cº,warms from 10 to 15ºC.The amount of heat transferred to the lake is
(Multiple Choice)
4.8/5  (33)
(33)
Use the following to answer question: 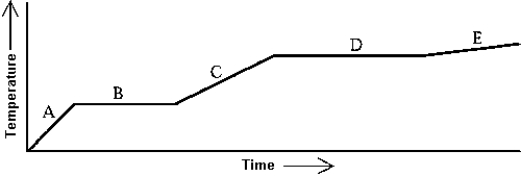 -Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The specific heat of the liquid can be found by
-Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The specific heat of the liquid can be found by
(Multiple Choice)
4.9/5  (26)
(26)
Use the following to answer the question: 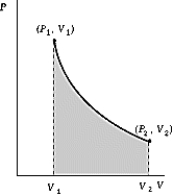 -One mole of an ideal gas ( = 5/3)expands adiabatically and quasistatically from a pressure P1 = 6 atm and a temperature of 50ºC to a pressure P2 = 4 atm.How much work is done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
-One mole of an ideal gas ( = 5/3)expands adiabatically and quasistatically from a pressure P1 = 6 atm and a temperature of 50ºC to a pressure P2 = 4 atm.How much work is done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
(Multiple Choice)
4.8/5  (35)
(35)
Use the following to answer the question:  -An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 50 kPa.How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
-An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 50 kPa.How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
(Multiple Choice)
4.8/5  (36)
(36)
A balloon contains gas at a pressure 1.2 atm (1 atm = 101.3 kPa)and has a volume of 0.10m3.More gas is pumped into the balloon at constant pressure until the volume is doubled.How much work is done by the pump?
(Multiple Choice)
4.8/5  (35)
(35)
You add 50 g of ice cubes to 125 g of water that is initially at 20ºC in a calorimeter of negligible heat capacity.When the system has reached equilibrium,how much of the ice remains?
(Multiple Choice)
4.8/5  (39)
(39)
When a substance goes directly from a solid state to a gaseous form,the process is known as
(Multiple Choice)
4.9/5  (29)
(29)
Use the following to answer question:  -Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The specific heat of the solid can be found by
-Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The specific heat of the solid can be found by
(Multiple Choice)
5.0/5  (39)
(39)
Use the following to answer the question: 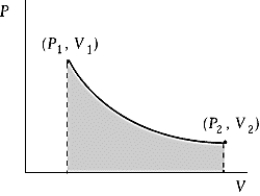 -An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
-An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
(Multiple Choice)
4.9/5  (32)
(32)
Use the following to answer the question:  -An ideal gas initially at 50ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
-An ideal gas initially at 50ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
(Multiple Choice)
4.8/5  (39)
(39)
The fact that most solids have molar heat capacities of approximately 3R,where R is the universal gas constant,is known as
(Multiple Choice)
4.7/5  (33)
(33)
Showing 41 - 60 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)