Exam 18: Heat and the First Law of Thermodynamics
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension83 Questions
Exam 3: Motion in Two and Three Dimensions60 Questions
Exam 4: Newtons Laws106 Questions
Exam 5: Applications of Newtons Laws73 Questions
Exam 6: Work and Energy60 Questions
Exam 7: Conservation of Energy56 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum92 Questions
Exam 9: Rotation105 Questions
Exam 10: Conservation of Angular Momentum66 Questions
Exam 11: Gravity84 Questions
Exam 12: Static Equilibrium and Elasticity58 Questions
Exam 13: Fluids77 Questions
Exam 14: Oscillations126 Questions
Exam 15: Wave Motion112 Questions
Exam 16: Superposition and Standing Waves87 Questions
Exam 17: Temperature and the Kinetic Theory of Gases78 Questions
Exam 18: Heat and the First Law of Thermodynamics100 Questions
Exam 19: The Second Law of Thermodynamics59 Questions
Exam 20: Thermal Properties and Processes50 Questions
Exam 21: The Electric Field I: Discrete Charge Distributions55 Questions
Exam 22: The Electric Field Ii: Continuous Charge Distributions64 Questions
Exam 23: Electric Potential87 Questions
Exam 24: Capacitance63 Questions
Exam 25: Electric Current and Direct-Current Circuits107 Questions
Exam 26: The Magnetic Field33 Questions
Exam 27: Sources of the Magnetic Field86 Questions
Exam 28: Magnetic Induction56 Questions
Exam 29: Alternating-Current Circuits106 Questions
Exam 30: Maxwells Equations and Electromagnetic Waves57 Questions
Exam 31: Properties of Light82 Questions
Exam 32: Optical Images106 Questions
Exam 33: Interference and Diffraction91 Questions
Exam 34: Wave Particle Duality and Quantum Physics140 Questions
Exam 35: Applications of the Schrodinger Equation42 Questions
Exam 36: Atoms113 Questions
Exam 37: Molecules39 Questions
Exam 38: Solids and the Theory of Conduction75 Questions
Exam 39: Relativity82 Questions
Exam 40: Nuclear Physics107 Questions
Exam 41: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
Two liquids,A and B,are mixed together,and the resulting temperature is 22 C.If liquid A has mass m and was initially at temperature 35 C,and liquid B has mass 3m and was initially at temperature 11 C,calculate the ratio of the specific heats of A divided by B.
(Multiple Choice)
4.8/5  (39)
(39)
The molar specific heat of copper is 24.5 J/(mol.K).The amount of heat needed to raise 126 g of copper by 2C is
(Multiple Choice)
4.8/5  (41)
(41)
A gas has a molar heat capacity at constant volume of 28.39 J/mol · K.Assume the equipartition theorem to be valid.How many degrees of freedom (including translational)are there for the molecules of this gas? (the ideal-gas law constant is R = 8.31 J/mol · K)
(Multiple Choice)
4.8/5  (35)
(35)
Besides Joule's classic experiment,another way of demonstrating the equivalence between mechanical energy and heat is the following: Put some lead shot into a glass tube,seal both ends of the tube,invert the tube quickly several times,and measure the temperature of the shot.If you assume that all the mechanical energy is converted into heat in the lead shot and that no heat is lost,what is the change in the temperature of the shot if the tube is 1.0 m long,there are 100 g of shot,and the tube is inverted 10 times? (The specific heat of lead is 0.128 kJ/kg · K.)
(Multiple Choice)
4.8/5  (34)
(34)
Body A has twice the mass and three times the specific heat of body B.They are supplied with equal amounts of heat.Body A experiences a temperature change T.What change in temperature is experienced by body B?
(Multiple Choice)
4.9/5  (38)
(38)
The quantity of heat absorbed by a body is determined from the formula Q = cm(Tf - Ti).A certain metal has a specific heat c = 0.21 cal/g Cº and a mass m = 25.6 g.The initial temperature is Ti = 34.6ºC,and the final temperature Tf = 54.6ºC.The quantity of heat absorbed is
(Multiple Choice)
4.8/5  (33)
(33)
An ideal gas is heated so that it expands at constant pressure.The gas does work W.What heat is added to the gas?
(Multiple Choice)
4.9/5  (35)
(35)
The pressure of a mass of air at 20 C is halved adiabatically.If the ratio of Cp to Cv for air is 1.41,calculate the resulting temperature.
(Multiple Choice)
4.8/5  (48)
(48)
A small water reactor recently installed at Podunk College is operating at the boiling point of water due to the malfunctioning of the cooling system.The operators observe that the water boils away at the rate of 10 L/min.If they assume that all of the reactor energy is absorbed in the water,the power developed by the reactor is approximately
(Multiple Choice)
4.9/5  (28)
(28)
An ideal gas undergoes a cyclic process in which total (positive)work W is done by the gas.What total heat is added to the gas in one cycle?
(Multiple Choice)
4.7/5  (36)
(36)
Use the following to answer question: 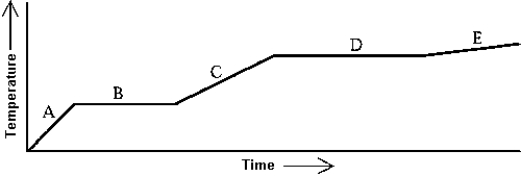 -Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated at constant volume.This process is shown in the graph.The specific heat at constant volume of the vapor can be found by
-Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated at constant volume.This process is shown in the graph.The specific heat at constant volume of the vapor can be found by
(Multiple Choice)
4.8/5  (37)
(37)
Use the following to answer the question: 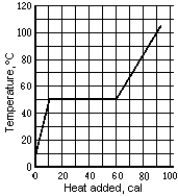 -The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The specific heat of the solid phase is
-The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The specific heat of the solid phase is
(Multiple Choice)
4.8/5  (40)
(40)
The temperature of water in the Gulf of Mexico can rise by about 20 F with the onset of the summer.However,the most significant temperature rise is confined to the top 2-3 meters of water.Assuming that it takes 45 days for the temperature to increase by 20 F,what is the average energy absorbed per day by water in a volume measuring 1 m 1 m 2.5 m?
(Multiple Choice)
4.9/5  (40)
(40)
A 4-kg mass of metal of unknown specific heat at a temperature of 600 C is dropped into 0.5 kg of ice and 0.5 kg of water both at 0 C.With no heat losses to the surroundings,the equilibrium temperature of the mixture is 85 C.Calculate the specific heat of the metal.
(Multiple Choice)
4.9/5  (26)
(26)
If the heat capacities of both ice and steam are 0.5 cal/g · Cº,the quantity of heat required to change 1 g of ice at -10ºC to steam at 120ºC is approximately
(Multiple Choice)
4.9/5  (31)
(31)
Use the following to answer the question:  -The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The melting point temperature is
-The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The melting point temperature is
(Multiple Choice)
4.8/5  (39)
(39)
Use the following to answer the question: 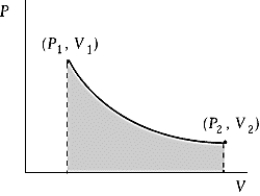 -An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasi-static,isothermal expansion until its pressure is reduced to 50 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
-An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasi-static,isothermal expansion until its pressure is reduced to 50 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
(Multiple Choice)
4.8/5  (38)
(38)
In a system composed of an ideal gas contained in a cylinder fitted with a piston,a reversible adiabatic expansion causes the temperature of the gas to drop because
(Multiple Choice)
4.9/5  (41)
(41)
How much internal energy is contained in 1 mole of monatomic gas at STP?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 81 - 100 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)